Co-precipitation Synthesis and Characterization Studies of Manganese Oxide Doped with Nickel for High-Performance Energy Storage Supercapacitor Application
DOI:
https://doi.org/10.48048/siam.2024.67005Abstract
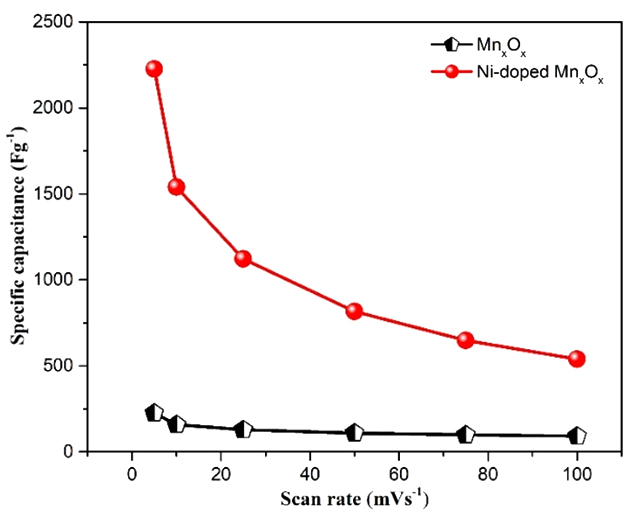
The advancement in nanotechnology research has facilitated the development of eco-friendly methods for the synthesis of nanoparticles. In this work, nickel (Ni)-doped manganese oxide was synthesized using the co-precipitation method. The prepared Ni-doped manganese oxide products have been characterized by X-ray powder diffraction, scanning electron microscopy (SEM), transmission electron microscope (TEM), and energy dispersive X-ray spectroscopy (EDS). The preliminary electrochemical characteristics include charge-discharge cycling, which improves the conductivity and capacitance of the high-performance aqueous asymmetrical supercapacitor. The CV analysis of the Ni-doped manganese oxide electrode demonstrated a distinctive pseudocapacitive behavior in 1 M KOH solution. The nickel (Ni) doped electrode has a higher specific capacitance value than the pure manganese oxide electrode, with a value of 2225.07 F×g-1 at a scan rate of 5 mV×s-1.
Keywords: Supercapacitor, Co-precipitation method, Nanotechnology, Synthesized, Electrochemical