Carbon Capture Based on Chemical Absorption: Process Design and Techno-Economic Assessments
Keywords:
NH3, DEA, MEA, Carbon capture, Chemical absorptionAbstract
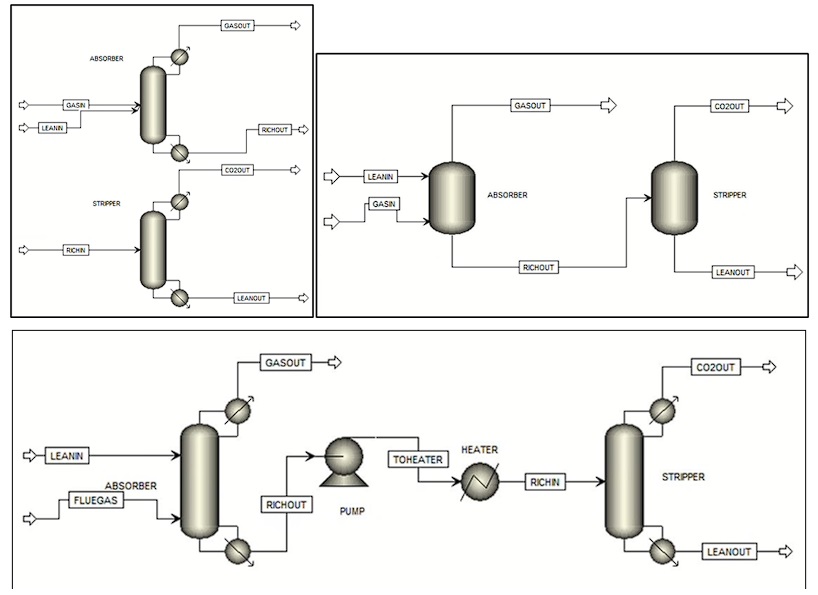
It is a widely accepted scientific fact that emissions of greenhouse gases, mostly Carbon dioxide (CO2) from fossil fuels, contribute to global warming. However, the world's energy industry continues to rely mostly on fossil fuels, which still provide an 85percent of the world's energy needs. The realization has set in that fossil fuels would remain the main energy source for many years due to the lack of economically viable sources of renewable power and the availability of cheap fuels including coal. Consequently, it is imperative to create technology that allows for the continued use of fossil fuels while reducing the amount of Carbon dioxide released into the environment. To minimize emissions into the atmosphere, CO2 from pollution sources should be captured. The theory behind several methods of CO2 collection will be examined in this study, and their benefits and drawbacks will be considered. After that, a selected separation method will be thoroughly examined by running simulations of the process utilizing the program As-pen Plus with three solvents, including NH3, DEA, and MEA. The effectiveness of the separation process was examined concerning operational circumstances. In contrast with other solvents, DEA stands out because of its increased CO2 removal efficiency and its decreased sensitivity to lean loading.
References
I. P. O. C. Change, “Climate change 2007: the physical science basis,” Agenda, vol. 6, no. 07, p. 333, 2007.
Y. S. Yu, Y. Li, H. F. Lu, R. F. Dong, Z. X. Zhang, and X. Feng, “Synergy pinch analysis of CO2 desorption process,” Industrial & engineering chemistry research, vol. 50, no. 24, pp. 13997–14007, 2011, doi: 10.3390/app7030286.
X. M. Wu, Y. S. Yu, C. Y. Zhang, G. X. Wang, and B. Feng, “Identifying the CO2 capture performance of CaCl2-supported amine adsorbent by the improved field synergy theory,” Industrial & Engineering Chemistry Research, vol. 53, no. 24, pp. 10225–10237, 2014, doi: 10.1021/ie500841n.
D. Kearns, H. Liu, and C. Consoli, “Technology readiness and costs of CCS,” Global CCS Institute, Brussels, Belgium, 2021.
J. Gibbins and H. Chalmers, “Carbon capture and storage,” Energy policy, vol. 36, no. 12, pp. 4317–4322, 2008, doi: 10.1016/j.enpol.2008.09.058.
E. Rubin, “Summary of the IPCC special report on carbon dioxide capture and storage,” 2006.
G. Qi et al., “High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules,” Energy & Environmental Science, vol. 4, no. 2, pp. 444–452, 2011, doi: 10.1039/C0EE00213E.
R. A. Gill, H. W. Polley, H. B. Johnson, L. J. Anderson, H. Maherali, and R. B. Jackson, “Nonlinear grassland responses to past and future atmospheric CO2,” Nature, vol. 417, no. 6886, pp. 279–282, 2002, doi: 10.1038/417279a.
T. C. Merkel, H. Lin, X. Wei, and R. Baker, “Power plant post-combustion carbon dioxide capture: An opportunity for membranes,” Journal of membrane science, vol. 359, no. 1–2, pp. 126–139, 2010, doi: 10.1016/j.memsci.2009.10.041.
Y. Peng, B. Zhao, and L. Li, “Advance in post-combustion CO2 capture with alkaline solution: a brief review,” Energy Procedia, vol. 14, pp. 1515–1522, 2012, doi: 10.1016/j.egypro.2011.12.1126.
C. Göpfert, C. Wamsler, and W. Lang, “Institutionalizing climate change mitigation and adaptation through city advisory committees: Lessons learned and policy futures,” City and Environment Interactions, vol. 1, p. 100004, 2019, doi: 10.1016/j.cacint.2019.100004.
D. Y. C. Leung, G. Caramanna, and M. M. Maroto-Valer, “An overview of current status of carbon dioxide capture and storage technologies,” Renewable and sustainable energy reviews, vol. 39, pp. 426–443, 2014, doi: 10.1016/j.rser.2014.07.093.
B. J. P. Buhre, L. K. Elliott, C. D. Sheng, R. P. Gupta, and T. F. Wall, “Progress Energy Combust,” Sci, vol. 31, no. 4, pp. 283–307, 2005, doi: 10.1016/j.pecs.2005.07.001.
L. L. Davies, K. Uchitel, and J. Ruple, “Understanding barriers to commercial-scale carbon capture and sequestration in the United States: An empirical assessment,” Energy Policy, vol. 59, pp. 745–761, 2013, doi: 10.1016/j.enpol.2013.04.033.
A. Raza, R. Gholami, R. Rezaee, V. Rasouli, and M. Rabiei, “Significant aspects of carbon capture and storage–A review,” Petroleum, vol. 5, no. 4, pp. 335–340, 2019, doi: 10.1016/j.petlm.2018.12.007.
P. Tcvetkov, A. Cherepovitsyn, and S. Fedoseev, “Public perception of carbon capture and storage: A state-of-the-art overview,” Heliyon, vol. 5, no. 12, p. e02845, 2019, doi: 10.1016/j.heliyon.2019.e02845.
M. Panahi and S. Skogestad, “Economically efficient operation of CO2 capturing process part I: Self-optimizing procedure for selecting the best controlled variables,” Chemical Engineering and Processing: Process Intensification, vol. 50, no. 3, pp. 247–253, 2011, doi: 10.1016/j.cep.2011.02.005.
S. Ziaii, G. T. Rochelle, and T. F. Edgar, “Dynamic modeling to minimize energy use for CO2 capture in power plants by aqueous monoethanolamine,” Industrial & Engineering Chemistry Research, vol. 48, no. 13, pp. 6105–6111, 2009, doi: 10.1021/ie801385q.
Y. A. C. Jande, M. Asif, S. M. Shim, and W.-S. Kim, “Energy minimization in monoethanolamine‐based CO2 capture using capacitive deionization,” International journal of energy research, vol. 38, no. 12, pp. 1531–1540, 2014, doi: 10.1002/er.3168.
S. Gantert and D. Möller, “Ultrasonic desorption of CO2–a new technology to save energy and prevent solvent degradation,” Chemical engineering & technology, vol. 35, no. 3, pp. 576–578, 2012, doi: 10.1002/ceat.201100395.
A. García-Abuín, D. Gómez-Díaz, and J. M. Navaza, “New processes for amine regeneration,” Fuel, vol. 135, pp. 191–197, 2014, doi: 10.1016/j.fuel.2014.06.067.
L. Zhao, R. Zhao, S. Deng, Y. Tan, and Y. Liu, “Integrating solar Organic Rankine Cycle into a coal-fired power plant with amine-based chemical absorption for CO2 capture,” International Journal of Greenhouse Gas Control, vol. 31, pp. 77–86, 2014, doi: 10.1016/j.ijggc.2014.09.025.
P. Luis, “Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives,” Desalination, vol. 380, pp. 93–99, 2016, doi: 10.1016/j.desal.2015.08.004.
M. Lucquiaud and J. Gibbins, “On the integration of CO2 capture with coal-fired power plants: A methodology to assess and optimise solvent-based post-combustion capture systems,” Chemical Engineering Research and Design, vol. 89, no. 9, pp. 1553–1571, 2011, doi: 10.1016/j.cherd.2011.03.003.
Y. Le Moullec, T. Neveux, A. Al Azki, A. Chikukwa, and K. A. Hoff, “Process modifications for solvent-based post-combustion CO2 capture,” International Journal of Greenhouse Gas Control, vol. 31, pp. 96–112, 2014, doi: 10.1016/j.ijggc.2014.09.024.
Z. Qing, G. Yincheng, and N. Zhenqi, “Experimental studies on removal capacity of carbon dioxide by a packed reactor and a spray column using aqueous ammonia,” Energy Procedia, vol. 4, pp. 519–524, 2011, doi: 10.1016/j.egypro.2011.01.083.
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.








