Activity of Carbon-Based Solid Acid Catalyst Derived from Palm Empty Fruit Bunch for Esterification of Palmitic Acid
Main Article Content
Abstract
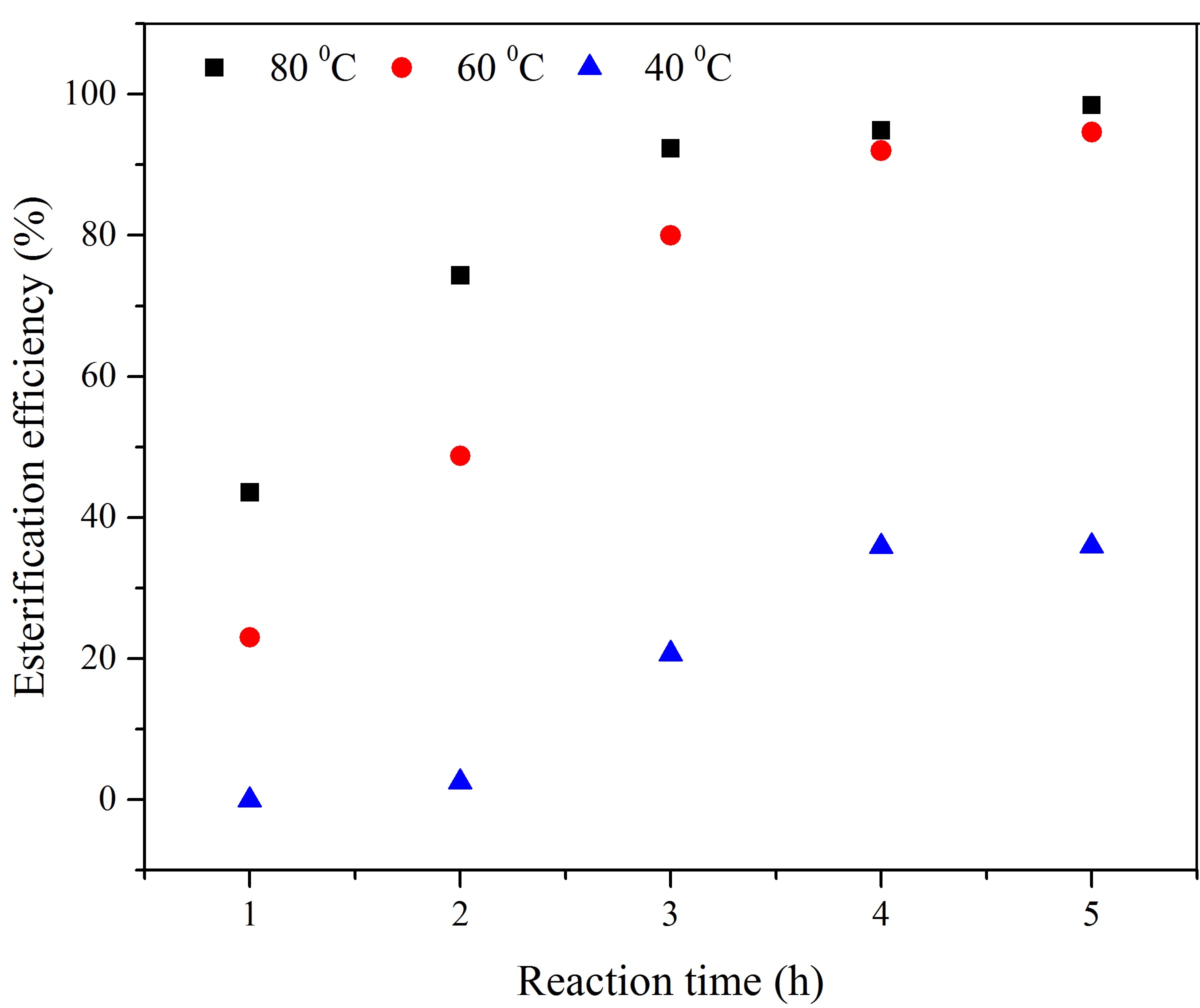
The activity of a heterogeneous solid acid catalyst derived from palm empty fruit bunch, synthesized through the direct in-situ H2SO4 impregnation was investigated for the esterification of palmitic acid. The prepared catalyst was characterized by scanning electron microscopy (SEM), Nitrogen adsorption and desorption isotherm, Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), and thermogravimetric analysis (TGA). It was also analyzed for acid density and elemental composition. The results revealed that the esterification efficiency increases with increasing reaction time, temperature, and methanol loading up to an optimum value. The catalyst showed an excellent activity resulting in >98% esterification efficiency using 5 wt% catalyst, a 6:1 methanol to palmitic acid molar ratio, at 80°C for 5 h in an open reflux reactor, for the reaction conditions. The catalyst was employed in three consecutive runs without considerable loss of the activity. The obtained high catalytic activity is attributed to the high acid density due to the presence of strong (SO3H) and weak (COOH, OH) acid sites in the hydrophobic carbon structure.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
2. Bennett JA, Wilson K, Lee AF. Catalytic applications of waste derived materials. Journal of Materials Chemistry A 2016;4(10):3617-37.
3. Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. Journal of the American Chemical Society 1938;60(2):309-19.
4. Coates J. Interpretation of infrared spectra: a practical approach. In: Meyers RA, editor. John Wiley & Sons Ltd, Chichester; 2000.
5. Dehkhoda AM. Developing biochar-based catalyst for biodiesel production [dissertation]. Vancouver, Canada: University of British Columbia; 2010.
6. Devi PBL, Gangadhar KN, Sai Prasad PS, Jagannadh B, Prasad RB. A glycerol‐based carbon catalyst for the preparation of biodiesel. ChemSusChem 2009; 2(7):617-20.
7. Fraile JM, García-Bordejé E, Roldán L. Deactivation of sulfonated hydrothermal carbons in the presence of alcohols: evidences for sulfonic esters formation. Journal of Catalysis 2012;289:73-9.
8. Fu J, Chen L, Lv P, Yang L, Yuan Z. Free fatty acids esterification for biodiesel production using self-synthesized macroporous cation exchange resin as solid acid catalyst. Fuel 2015;154:1-8.
9. Fu X, Li D, Chen J, Zhang Y, Huang W, Zhu Y, Yang J, Zhang C. A microalgae residue based carbon solid acid catalyst for biodiesel production. Bioresource Technology 2013;146:767-70.
10. Fu Z, Wan H, Hu X, Cui Q, Guan G. Preparation and catalytic performance of a carbon-based solid acid catalyst with high specific surface area. Reaction Kinetics, Mechanisms and Catalysis 2012;107(1): 203-13.
11. Han X-X, Chen K-K, Yan W, Hung C-T, Liu L-L, Wu P-H, Lin K-C, Liu S-B. Amino acid-functionalized heteropolyacids as efficient and recyclable catalysts for esterification of palmitic acid to biodiesel. Fuel 2016;165:115-22.
12. Hara M. Biomass conversion by a solid acid catalyst. Energy and Environmental Science 2010;3(5):601-7.
13. Konwar LJ, Das R, Thakur AJ, Salminen E, Mäki-Arvela P, Kumar N, Mikkola J-P, Deka D. Biodiesel production from acid oils using sulfonated carbon catalyst derived from oil-cake waste. Journal of Molecular Catalysis A: Chemical 2014;388:167-76.
14. Liu R-L, Gao X-Y, An L, Ma J, Zhang J-F, Zhang Z-Q. Fabrication of magnetic carbonaceous solid acids from banana peel for the esterification of oleic acid. RSC Advances 2015;5(114):93858-66.
15. Malins K, Brinks J, Kampars V, Malina I. Esterification of rapeseed oil fatty acids using a carbon-based heterogeneous acid catalyst derived from cellulose. Applied Catalysis A: General 2016;519:99-106.
16. Nakajima K, Hara M, Hayashi S. Environmentally benign production of chemicals and energy using a carbon‐based strong solid acid. Journal of the American Ceramic Society 2007;90(12):3725-34.
17. Ngaosuwan K, Goodwin JG, Prasertdham P. A green sulfonated carbon-based catalyst derived from coffee residue for esterification. Renewable Energy 2016;86:262-9.
18. Russo PA, Antunes MM, Neves P, Wiper PV, Fazio E, Neri F, Barreca F, Mafra L, Pillinger M, Pinna N, Valente AA. Solid acids with SO3H groups and tunable surface properties: versatile catalysts for biomass conversion. Journal of Materials Chemistry A 2014;2(30):11813-24.
19. Saravanan K, Tyagi B, Shukla RS, Bajaj HC. Solvent free synthesis of methyl palmitate over sulfated zirconia solid acid catalyst. Fuel 2016;165:298-305.
20. Savaliya ML, Dholakiya BZ. A simpler and highly efficient protocol for the preparation of biodiesel from soap stock oil using a BBSA catalyst. RSC Advances 2015;5(91):74416-24.
21. Su F, Guo Y. Advancements in solid acid catalysts for biodiesel production. Green Chemistry 2014;16(6): 2934-57.
22. The National Standard of the People's Republic of China. GB/T 5530: Animal and vegetable fats and oils determination of acid value. Standard Press of China: Beijing; 2005.
23. Thushari P, Babel S. Biodiesel production from waste palm oil using palm empty fruit bunch-derived novel carbon acid catalyst. Journal of Energy Resources Technology 2018;140(3):032204.
24. Visvanathan C, Chiemchaisri C. Management of Agricultural Wastes and Residues in Thailand: Wastes to Energy Approach. Asian Institute of Technology: Thailand; 2008.
25. Zeng D, Zhang Q, Chen S, Liu S, Wang G. Synthesis of porous carbon-based solid acid from rice husk for esterification of fatty acids. Microporous and Mesoporous Materials 2016;219:54-8.
26. Zhang Z, Huang H, Ma X, Li G, Wang Y, Sun G, Teng Y, Yan R, Zhang N, Li A. Production of diacylglycerols by esterification of oleic acid with glycerol catalyzed by diatomite loaded SO42−/TiO2. Journal of Industrial and Engineering Chemistry 2017;53:307-16.
27. Zhou Y, Niu S, Li J. Activity of the carbon-based heterogeneous acid catalyst derived from bamboo in esterification of oleic acid with ethanol. Energy Conversion and Management 2016;114:188-96.