Catalytic Ozonation using Iron-Doped Water Treatment Sludge as a Catalyst for Treatment of Phenol in Synthetic Wastewater DOI: 10.32526/ennrj.17.2.2019.15
Main Article Content
Abstract
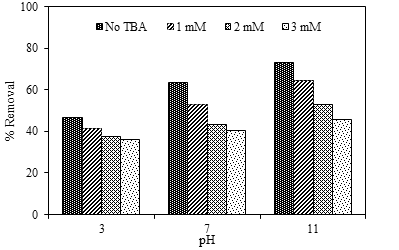
In this study, iron (Fe)-doped water treatment sludge, designated as Fe/WTS, was prepared by a hydrothermal method using phosphoric acid and impregnation with ferric nitrate. The results from X-ray diffraction (XRD) confirmed the presence of Fe loaded on the WTS support, while Brunauer-Emmett-Teller (BET) analysis indicated an increase of specific surface area of the WTS from 37.37 m2/g to 118.51 m2/g after acid modification. The Fe/WTS was successfully used as a catalyst in catalytic ozonation for degradation of phenol in synthetic wastewater. Factors affecting phenol removal efficiency including reaction time, pH, catalyst dosage, and Fe content were investigated. At the optimum condition, i.e., reaction time of 120 min, pH of 11, catalyst dosage of 1 g/L, and Fe content of 2% (w/w), the removal efficiency of phenol was 99.16% which was higher than that of sole ozonation (44.61%). The results of kinetic analyses indicated that the reactions of catalytic ozonation in the presence of Fe/WTS and WTS catalysts followed pseudo-first order kinetic model with rate constants of 0.0362 and 0.0065 min-1, respectively, while that of sole ozone was 0.0046 min-1. This finding presented the potential use of Fe/WTS as a novel catalyst for catalytic ozonation.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
2. Ayodele OB, Lim LK, Hameed BH. Degradation of phenol in photo-fenton process by phosphoric acid modified kaolin supported ferric-oxalate catalyst: optimization and kinetic modelling. Chemical Engineering Journal 2012;197:181-92.
3. Belver C, Munoz M, Vicente M. Chemical activation of a kaolinite under acid and alkaline conditions. Chemical Matter 2002;14:2033-43.
4. Chen W, Li X, Pan Z, Ma S, Li L. Effective mineralization of dicrophenac by catalytic ozonation using Fe-MCM-41 catalyst. Chemical Engineering Journal 2016; 304:594-601.
5. Dai C, Zhang A, Luo L, Zhang X, Liu M, Wang J. Hollow zeolite-encapsulated Fe-Cu bimetallic catalytic for phenol degradation. Catalyst Today 2017;297:335-43.
6. Farzadkia M, Shahamat YD, Nasseri S, Mahvi AH, Gholami M, Shahryari A. Catalytic ozonation of phenolic wastewater: Identification and toxicity of intermediates. Hindawi Publishing Corporation Journal of Engineering 2014;1-10.
7. He K, Dong Y, Li M, Zhen YL, Zhang AM, Zheng YC. Catalytic ozonation of phenol in water with natural brucite and magnesia. Journal of Hazardous Materials 2008;159:587-92.
8. Kizinievic O, Zurauskiene R, Kizinievic V, Zurauskas R. Utilisation of sludge waste from water treatment for ceramic products. Construction and Building Materials 2013;41:464-73.
9. Lan B, Huang R, Li L, Ma J, Yan H, Liao G, Wang X, Zhang Q. Catalytic ozonation of p-chlorobenzoic acid in aqueous solution using Fe-MCM-41 as catalyst. Chemical Engineering Journal 2013;219:346-54.
10. Li G, Zhai Y, Ma H, Wang B. Degradation of cationic dye methylene blue by ozonation assisted with kaolin. Applied Clay Science 2009;46:226-9.
11. Li H, Ji J, Cheng C, Liang K. Preparation of phenol-formaldehyde resin-coupled TiO2 and study of photocatalytic activity during phenol degradation under sunlight. Journal of Physics and Chemistry of Solids 2018a;122:25-30.
12. Li X, Chen W, Tang Y, Li L. Relationship between the structure of Fe-MCM-48 and its activity in catalytic ozonation for diclofenac mineralization. Chemosphere 2018b;206:615-21.
13. Liotta LF, Gruttadauria M, Carlo GD, Perrini G, Librando V. Heterogeneous catalytic degradation of phenolic substrates: catalysts activity. Journal of Hazardous Materials 2009;162:588-606.
14. Ma H, Zhuo Q, Wang B. Electro-catalytic degradation of methylene blue wastewater assisted by Fe2O3-modified kaolin. Chemical Engineering Journal 2009;155:248-53.
15. Panda AK, Mishra BG, Mishra DK, Singh RK. Effect of sulfuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids and Surface A: Physicochemical and Engineering Aspect 2010;363:98-104.
16. Peng J, Lai L, Jiang X, Jiang W, Lai B. Catalytic ozonation of succinic acid in aqueous solution using the catalyst of Ni/Al2O3 prepared by electroless planting-calcination method. Separation and Purification Technology 2018;195:138-48.
17. Qi F, Xu B, Chen Z, Ma J, Sun D, Zhang L, Wu F. Ozonation catalyzed by the raw bauxite for the degradation of 2,4,6 trichloroanisole in drinking water. Journal of Hazardous Materials 2009;168:246-52.
18. Qi F, Xu B, Zhao L, Chen Z, Zhang L, Sun D, Ma J. Comparison of the efficiency and mechanism of catalytic ozonation of 2,4,6 trichloroanisole by iron and manganese modified bauxite. Applied Catalysis B: Environmental 2012;121-122:171-81.
19. Rice EW, Baird RB, Eaton AD. Standard Methods for the Examination of Water and Wastewater. 23rd ed. American Public Health Association, American Water Works Association, Water Environment Federation; 2017.
20. Sirifhognugoon S. The Use of Water Treatment Sludge as Zinnia (Zinnia elegans) Growing Media [dissertation]. Bangkok, Thailand: Kasetsart University; 2000.
21. Shahamat YD, Farzadkia M, Nasseri S, Mahvi AH, Gholami M, Shahryari A. Magnetic heterogeneous catalytic ozonation: a new removal method for phenol in industrial wastewater. Journal of Environmental Health Science and Engineering 2014;12(50):1-12.
22. Shahidi D, Roy R, Azzouz A. Advances in catalytic oxidation of organic pollutants-prospects for through mineralization by natural clay catalyst. Applied Catalysis B: Environmental 2015;174-175:277-92.
23. Vinitnantharat S, Kositchaiyong S, Chairakorn S. Removal of fluoride in aqueous solution by adsorption on acid activated water treatment sludge. Applied Clay Science 2010;256:5458-62.
24. von Gunten U. Ozonation of drinking water: part I. oxidation kinetics and product formation. Water Research 2003;37:1443-67.
25. Wang J, Bai Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chemical Engineering Journal 2017;312:79-98.
26. Zhang F, Wu K, Zhou H, Hu Y, Sergei P, Wu H. Ozonation of aqueous phenol catalyzed by biochar produced from sludge obtained in treatment of coking wastewater. Journal of Environmental Management 2018;224:76-386.
27. Zhao L, Ma J, Sun ZZ, Zhai XD. Preliminary kinetic study on the degradation of nitrobenzene by modified ceramic honeycomb-catalytic ozonation in aqueous solution. Journal of Hazardous Materials 2009;161(2-3):988-94.