A Simple Method for Synthesis of Triamine-SiO2 Material toward Aqueous Nitrate Adsorption DOI: 10.32526/ennrj.17.4.2019.31
Main Article Content
Abstract
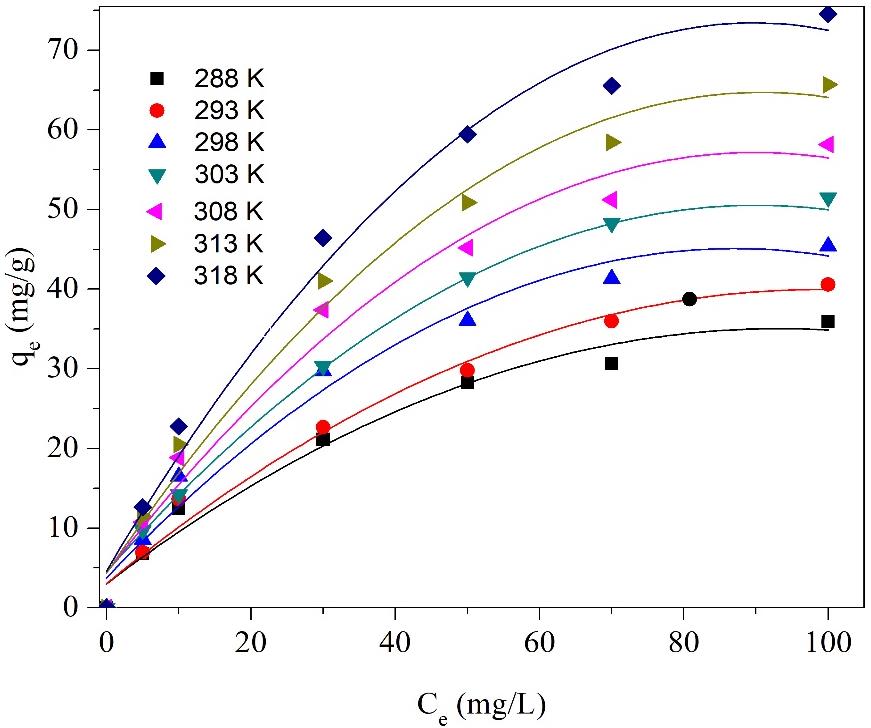
This study describes a facile route for preparation of mesoporous silica at ambient condition using cheap and available commercial SiO2 precursor. The mesoporous material was then loaded with amine (Amine-P-SiO2) and applied for nitrate removal in aqueous solution. The materials were characterized by XRD, TGA, FTIR, and SEM to explore the properties. Effects of pH, nitrate concentration, adsorbent dosage, and temperature on the nitrate adsorption capacity were investigated. Amine-P-SiO2 material was superior to commercial adsorbent (Akualite A420) for nitrate adsorption with capacity reaching 32.5 mg/g and relatively stable after 10 cycles of adsorption-desorption. Moreover, the adsorption follows Langmuir model, proving that this chemical adsorption could effectively remove nitrate from aqueous solution for water and advanced wastewater treatment applications.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
2. Bahadori E, Compagnoni M, Tripodi A, Freyria F, Armandi M, Bonelli B, Ramis G, Rossetti I. Photoreduction of nitrates from waste and drinking water. Materials Today: Proceedings 2018;5(9, Part 2):17404-13.
3. Banu HT, Meenakshi S. Synthesis of a novel quaternized form of melamine - formaldehyde resin for the removal of nitrate from water. Journal of Water Process Engineering 2017;16:81-9.
4. Battas A, Gaidoumi AE, Ksakas A, Kherbeche A. Adsorption study for the removal of nitrate from water using local clay. The Scientific World Journal 2019;2019:1-10.
5. Bhatnagar A, Sillanpää M. A review of emerging adsorbents for nitrate removal from water. Chemical Engineering Journal 2011;168(2):493-504.
6. Duranoğlu D, Trochimczuk AW, Beker U. Kinetics and thermodynamics of hexavalent chromium adsorption onto activated carbon derived from acrylonitrile-divinylbenzene copolymer. Chemical Engineering Journal 2012;187:193-202.
7. Gandhi MR, Kalaivani G, Meenakshia S. Sorption of chromate and fluoride onto duolite a 171 anion exchange resin - a comparative study. Elixir Pollution 2011;32:2034-40.
8. Garcia-Segura S, Lanzarini-Lopes M, Hristovski K, Westerhoff P. Electrocatalytic reduction of nitrate: Fundamentals to full-scale water treatment applications. Applied Catalysis B: Environmental 2018;236:546-68.
9. Hamoudi S, Saad R, Belkacemi K. Adsorptive removal of phosphate and nitrate anions from aqueous solutions using ammonium-functionalized mesopo-rous silica. Industrial and Engineering Chemistry Research 2007;46(25):8806-12.
10. Ibrahim DM, El-Hemaly SA, Abdel-Kerim FM. Study of rice-husk ash silica by infrared spectroscopy. Thermochimica Acta 1980;37(3):307-14.
11. Kalaruban M, Loganathan P, Shim WG, Kandasamy J, Naidu G, Nguyen TV, Vigneswaran S. Removing nitrate from water using iron-modified Dowex 21K XLT ion exchange resin: Batch and fluidised-bed adsorption studies. Separation and Purification Technology 2016;158:62-70.
12. Katal R, Baei MS, Rahmati HT, Esfandian H. Kinetic, isotherm and thermodynamic study of nitrate adsorption from aqueous solution using modified rice husk. Journal of Industrial and Engineering Chemistry 2012;18(1):295-302.
13. Lazar L, Bandrabur B, Tataru-Fărmuş R-E, Drobotă M, Bulgariu L, Gutt G. FTIR analysis of ion exchange resins with application in permanent hard water softening. Environmental Engineering and Management Journal 2014;13(9):2145-52
14. Lee B, Bao L-L, Im H-J, Dai S, Hagaman EW, Lin JS. Synthesis and characterization of organic-inorganic hybrid mesoporous anion-exchange resins for perrhenate (ReO4-) anion adsorption. Langmuir 2003;19(10):4246-52.
15. Loganathan P, Vigneswaran S, Kandasamy J. Enhanced removal of nitrate from water using surface modification of adsorbents: A review. Journal of Environmental Management 2013;131:363-74.
16. Martinez J, Ortiz A, Ortiz I. State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates. Applied Catalysis B: Environmental 2017;207:42-59.
17. Milmile SN, Pande JV, Karmakar S, Bansiwal A, Chakrabarti T, Biniwale RB. Equilibrium isotherm and kinetic modeling of the adsorption of nitrates by anion exchange Indion NSSR resin. Desalination 2011;276(1):38-44.
18. Nujić M, Milinković D, Habuda-Stanić M. Nitrate removal from water by ion exchange. Croatian Journal of Food Science and Technology 2017;9(2):182-6.
19. Phan PT, Nguyen TT, Nguyen NH, Padungthon S. Triamine-bearing activated rice husk ash as an advanced functional material for nitrate removal from aqueous solution. Water Science and Technology 2018;79(5):850-6.
20. Saad R, Belkacemi K, Hamoudi S. Adsorption of phosphate and nitrate anions on ammonium-functionalized MCM-48: Effects of experimental conditions. Journal of Colloid and Interface Science 2007;311(2):375-81.
21. Safia H, Abir EN, Maissa B, Khaled B. Adsorptive removal of nitrate and phosphate anions from aqueous solutions using functionalised SBA‐15: Effects of the organic functional group. The Canadian Journal of Chemical Engineering 2012;90(1):34-40.
22. Singh NB, Nagpal G, Agrawal S, Rachna. Water purification by using Adsorbents: A Review. Environmental Technology and Innovation 2018;11:187-240.
23. Song W, Gao B, Xu X, Wang F, Xue N, Sun S, Song W, Jia R. Adsorption of nitrate from aqueous solution by magnetic amine-crosslinked biopolymer based corn stalk and its chemical regeneration property. Journal of Hazardous Materials 2016;304:280-90.
24. Sowmya A, Meenakshi S. Removal of nitrate and phosphate anions from aqueous solutions using strong base anion exchange resin. Desalination and Water Treatment 2013;51(37-39):7145-56.
25. Thanh NT. Amine-bearing activated rice husk ash for CO2 and H2S gas removals from biogas. KKU Engineering Journal 2016;43(S3):396-8.
26. Tugaoen HON, Garcia-Segura S, Hristovski K, Westerhoff P. Challenges in photocatalytic reduction of nitrate as a water treatment technology. Science of the Total Environment 2017;599:1524-51.
27. Tyagi S, Rawtani D, Khatri N, Tharmavaram M. Strategies for nitrate removal from aqueous environment using nanotechnology: A Review. Journal of Water Process Engineering 2018;21:84-95.
28. Wołowicz A, Hubicki Z. Sorption of palladium(II) complexes onto the styrene-divinylbenzene anion exchange resins. Chemical Engineering Journal 2009;152(1):72-9.
29. Yousef RI, El-Eswed B, Ala’a H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chemical Engineering Journal 2011;171(3):1143-9.