The Alternating Growth of Bacteria within a Consortium During Desulfurization of Coal 10.32526/ennrj/20/202100145
Main Article Content
Abstract
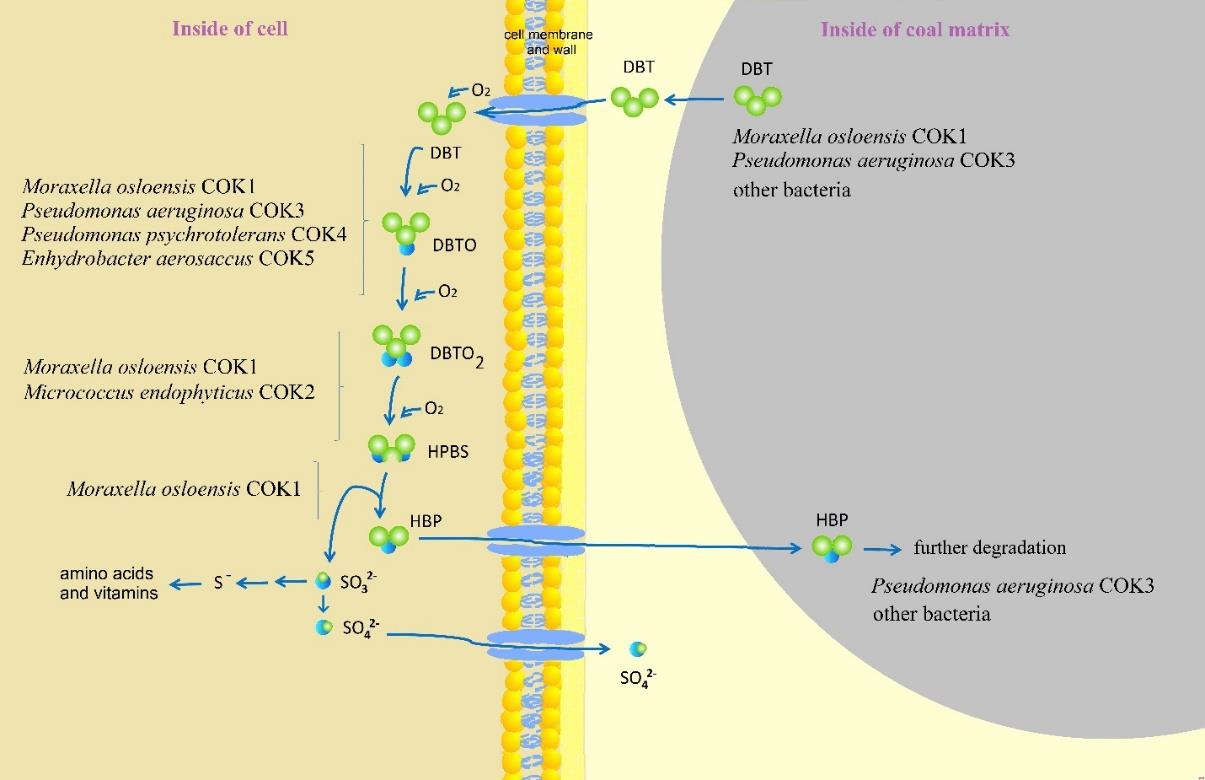
Efforts to reduce organic sulfur in coal are taken through biodesulfurization by using desulfurization bacteria to release covalently-bound sulfur from the coal matrix. Coal is a complex hydrocarbon material that requires collaboration from more than one type of bacteria in a consortium for desulfurization. The current study shows how the individual members of a bacterial consortium obtained directly from coal samples grew on the coal. Mineral medium containing sub-bituminous coal with a concentration of 10%, 15%, and 20% served as a carbon source and the only sulfur to support the consortium's growth. The examination included growth patterns, concentrations of dibenzothiophene as an organic sulfur representative, pH, and sulfate concentration as the sulfur product released into the medium. The growth of individual members of the consortium was observed for 336 h. The consortium grew in all three coal concentrations with slightly different cell growth patterns and the release of dibenzothiophene. Members of the consortium grew alternately and overlapped, which showed possible linkages or dependence on products and existence from the growth of other members. The existence of the primary strain Moraxella osloensis COK1 indicated that they played a role in the activities and growth of other members. The alternating growth is discussed to produce a hypothetical illustration of how several other members play in using sulfur in a well-known desulfurization pathway. In conclusion, this study provides a deeper insight into the value of consortium members individually but growing together while swarming coal as a complex resource to become low-sulfur coal.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology 1990;215:403-10.
Burke V, Wiley AJ. Bacteria in coal. Journal of Bacteriology 1937;34:475-81.
Chakraborty J, Das S. Characterization of the metabolic pathway and catabolic gene expression in biphenyl degrading marine bacterium Pseudomonas aeruginosa JP-11. Chemosphere 2016;144:1706-14.
Chen S, Zhao C, Liu Q, Zhang X, Sun S, Zang M. Biodesulfurization of diesel oil in oil - water two phase reaction system by Gordonia sp SC-10. Biotechnology Letters 2019;41:547-54.
Colmer AR, Temple KL, Hinkle ME. An iron-oxidizing bacterium from the acid drainage of some bituminous coal mines. Journal of Bacteriology 1949;59:317-28.
Constanti M, Giralt J, Bordons A. Desulphurization of dibenzothiophe by bacteria. World Journal of Microbiology and Biotechnology 1994;10:510-16.
Etemadifar Z, Emtiazi G, Christofi N. Enhanced desulfurization activity in protoplast transformed Rhodococcus erythropolis. American-Eurasian Journal of Agriculture and Environmental Science 2008;3:795-801.
Ghosh P, Mukherji S. Degradation of carbazole, fluorene, dibenzothiophene and their mixture by P. aeruginosa RS1 in petroleum refinery wastewater. Journal of Water Process Engineering 2020;37:Article No. 101454.
Gunam IBW, Yaku Y, Hirano M, Yamamura K, Tomita F, Sone T, et al. Biodesulfurization of alkylated forms of dibenzothiophene and benzothiophene by Sphingomonas subarctica T7b. Journal of Bioscience and Bioengineering 2006;101:322-7.
Hamer U, Marschner B, Brodowski S, Amelung W. Interactive priming of black carbon and glucose mineralisation. Organic Geochemistry 2004;35:823-30.
Hirschler A, Carpito C, Maurer L, Zumsteg J, Villettte C, Heintz D, et al. Biodesulfurization induces reprogramming of sulfur metabolism in Rhodococcus qingshengii IGTS8: Proteomics and untargeted metabolomics. Microbiolgy Spectrum 2021; 9:e00692-21.
Jatoi AS, Aziz S, Soomro SA. Effect of native microorganism Rhodococcus spp SL-9 for dibenzothiophene degradation and its application towards clean coal approach. Cleaner Engineering and Technology 2021;3:Article No. 100126.
Kilbane JJ. Biodesulfurization: How to make it work ? Arabian Journal for Science and Engineering 2016;42:1-9.
Kotelnikov VI, Saryglar CA, Chysyma RB. Microorganisms in coal desulfurization (Review). Applied Biochemistry and Microbiology 2020;56:521-5.
Li L, Shen X, Zhao C, Liu Q, Liu X, Wu Y. Biodegradation of dibenzothiophene by efficient Pseudomonas sp. LKY-5 with the production of a biosurfactant. Ecotoxicology and Environmental Safety 2019;176:50-7.
Li M, Wang T, Simoneit BRT, Shi S, Zhang L, Yang F. Qualitative and quantitative analysis of dibenzothiophene, its methylated homologues, and benzonaphthothiophenes in crude oils, coal, and sediment extracts. Journal of Chromatography A 2012; 1233:126-36.
Makgato SS, Chirwa EMN. Recent developments in reduction of sulphur emissions from selected Waterberg coal samples used in South African power plants. Journal of Cleaner Production 2020;276:Article No. 123192.
Martín-Cabello G, Terrón-González L, Ferrer M, Santero E.Identification of a complete dibenzothiophene bio-desulfurization operon and its regulator by functional metagenomics. Environmental Microbiology 2020;22:91-106.
McLeish AG, Vick SHW, Grigore M, Pinetown KL, Midgley DJ, Paulsen IT. Adherent microbes in coal seam environments prefer mineral-rich and crack-associated microhabitats. International Journal of Coal Geology 2021;234:Article No. 103652.
Mishra S, Pradhan N, Panda S, Akcil A. Biodegradation of dibenzothiophene and its application in the production of clean coal. Fuel Processing Technology 2016;152:325-42.
Mohebali G, Ball AS. Biodesulfurization of diesel fuels - Past, present and future perspectives. International Biodeterioration and Biodegradation 2016;110:163-80.
Nassar HN, Amr SSA, El-gendy NS. Biodesulfurization of refractory sulfur compounds in petro-diesel by a novel hydrocarbon tolerable strain Paenibacillus glucanolyticus HN. Environmental Science and Pollution Research 2021;28:8102-16.
Sousa JPM, Ferreira P, Neves RPP, Ramos MJ, Fernandes PA. The bacterial 4S pathway - an economical alternative for crude oil desulphurization that reduces CO2 emissions. Green Chemistry 2020;22:7604-21.
Strąpoć D, Picardal FW, Turich C, Schaperdoth I, Macalady JL, Lipp JS, et al. Methane-producing microbial community in a coal bed of the Illinois Basin. Applied and Environmental Microbiology 2008;74:2424-32.
Su X, Zhao W, Xia D. The diversity of hydrogen ‑ producing bacteria and methanogens within an in situ coal seam. Biotechnology for Biofuels 2018;11:1-18.
Taiwo J, Oghenekume O, Edeki G, Keith A. Bacterial degradation of coal discard and geologically weathered coal. International Journal of Coal Science and Technology 2020;7:405-16.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 2013;30:2725-9.
Vick SHW, Gong S, Sestak S, Vergara TJ, Pinetown KL, Li Z, et al. Who eats what? Unravelling microbial conversion of coal to methane. FEMS Microbiology Ecology 2019;95(7):Article Code. fiz093.
Wang B, Yu Z, Zhang Y, Zhang H. Microbial communities from the Huaibei Coalfield alter the physicochemical properties of coal in methanogenic bioconversion. International Journal of Coal Geology 2019;202:85-94.
Wasi S, Tabrez S, Ahmad M. Use of Pseudomonas spp. for the bioremediation of environmental pollutants: A review. Environmental Monitoring and Assessment 2013;185: 8147-55.
Yossifova MG, Valčeva SP, Nikolova SF. Exogenic microbial activity in coals. Fuel Processing Technology 2011;92:825-35.