M2+(Ni, Cu, Zn)/Al-LDH Composites with Hydrochar from Rambutan Peel and Study the Adsorption Efficiency for Organic Dyes 10.32526/ennrj/20/202100218
Main Article Content
Abstract
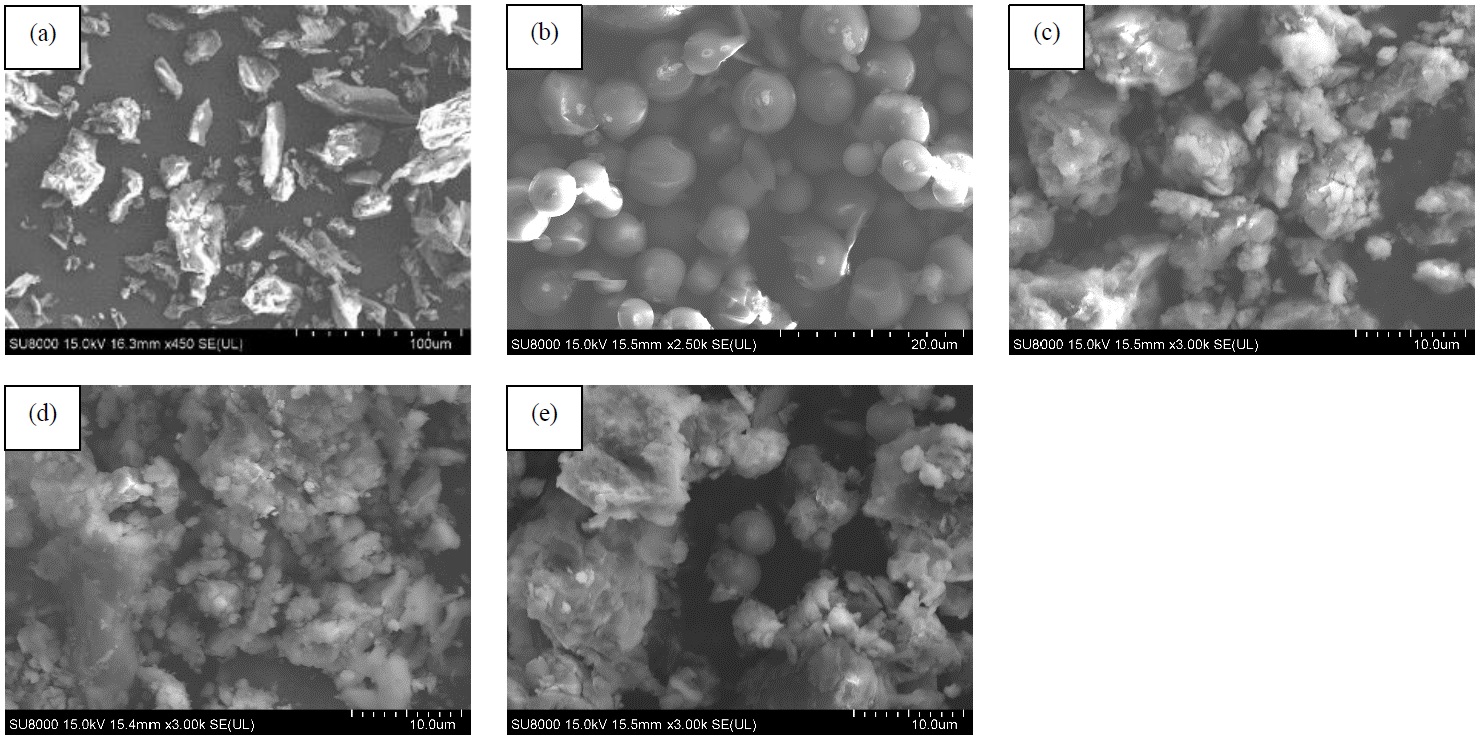
Ni/Al LDH, Cu/Al LDH, and Zn/Al LDH were composed with rambutan peel hydrochar (Hc) and the materials were applied as adsorbent for the removal of methylene blue from aqueous solution, measured using UV-Vis Spectrophotometric method. The preparation of the LDH-Hc composites were proven by XRD, FT-IR, and SEM analysis which showed similar characteristics of the LDH-Hc composites with pure LDH and hydrochar. The methylene blue removal efficiency was optimized by various parameters including adsorption selectivity, adsorption regeneration, pH, contact time, adsorption concentration, and temperature. The adsorption study analysis proved that LDH composited with rambutan peel hydrochar had a selective ability for methylene blue. Zn/Al-hydrochar had the most stable adsorption regeneration ability and adsorbed MB easily after seven regeneration cycles using water solvents and ultrasonic devices. Ni/Al-hydrochar and Cu/Al-hydrochar were effective up to five regeneration cycles for MB removal. The adsorption results showed that the optimal pH for MB adsorption was at pH 6 with an equilibrium adsorption contact time of 100 min and a tendency to follow the pseudo second order kinetic model. Parameter data of concentration and temperature of adsorption was determined using Langmuir and Freundlich equations. The results showed that the adsorption matched the Freundlich isotherm model with the adsorption capacity (qm) of Ni/Al-Hc, Cu/Al-Hc, and Zn/Al-Hc adsorbents reaching 144.928, 175.439, 217.391 mg/g, respectively, with the adsorption process taking place continuously, spontaneously, and endothermically.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Abdellaoui K, Pavlovic I, Barriga C. Nanohybrid layered double hydroxides used to remove several dyes from water. ChemEngineering 2015;3(2):Article No. 41.
Adeyemo AA, Adeoye IO, Bello OS. Adsorption of dyes using different types of clay: A review. Applied Water Science 2017;7(2):543-68.
Ahmad A, Jini D, Aravind M, Parvathiraja C, Ali R, Kiyani MZ, et al. A novel study on synthesis of egg shell based activated carbon for degradation of methylene blue via photocatalysis. Arabian Journal of Chemistry 2020;13(12):8717-22.
Al-Ghouti MA, Al-Absi RS. Mechanistic understanding of the adsorption and thermodynamic aspects of cationic methylene blue dye onto cellulosic olive stones biomass from wastewater. Scientific Reports 2020;10(1):Article No. 15928.
Alagha O, Manzar MS, Zubair M, Anil I, Mu’azu ND, Qureshi A. Magnetic Mg-Fe/LDH intercalated activated carbon composites for nitrate and phosphate removal from wastewater: Insight into behavior and mechanisms. Nanomaterials 2020;10(7):Article No. 1361
Alinezhad H, Zabihi M, Kahfroushan D. Design and fabrication the novel polymeric magnetic boehmite nanocomposite (boehmite@ Fe3O4@PLA@SiO2) for the remarkable competitive adsorption of methylene blue and mercury ions. Journal of Physics and Chemistry of Solids 2020;144:Article No. 109515.
Alrozi R, Zamanhuri NA, Osman MS. Removal of methylene blue from aqueous solution by adsorption onto NaOH-treated rambutan peel. 2012 IEEE Business, Engineering and Industrial Applications Colloquium (BEIAC) 2012;5:92-7.
Alver E, Metin AÜ, Brouers F. Methylene blue adsorption on magnetic alginate/rice husk bio-composite. International Journal of Biological Macromolecules 2020;154:104-13.
Badri A, Alvarez-Serrano I, Luisa López M, Ben Amara M. Sol-gel synthesis, magnetic and methylene blue adsorption properties of lamellar iron monophosphate KMgFe(PO4)2. Inorganic Chemistry Communications 2020;121:Article No. 108217.
Bezerra BGP, Bieseki L, de Mello MIS, da Silva DR, Rodella CB, Pergher S. Memory effect on a LDH/zeolite a composite: An XRD in situ study. Materials 2021;14(9):Article No. 2102.
Binh QA, Nguyen HH. Investigation the isotherm and kinetics of adsorption mechanism of herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) on corn cob biochar. Bioresource Technology Reports 2020;11:Article No. 100520.
Castro JDS, das Virgens CF. Thermal decomposition of Nephelium lappaceum L. peel: Influence of chemical pretreatment and evaluation of pseudo-components by Fraser-Suzuki function. Journal of Thermal Analysis and Calorimetry 2019;138(5):3541-9.
Chopra M, Drivjot, Amita. Adsorption of dyes from aqueous solution using orange peels: Kinetics and equilibrium. Journal of Advanced Laboratory Research in Biology 2012;3(1):1-8.
Dang W, Zhang J, Nie H, Wang F, Tang X, Wu N, et al. Isotherms, thermodynamics and kinetics of methane-shale adsorption pair under supercritical condition: Implications for understanding the nature of shale gas adsorption process. Chemical Engineering Journal 2020;383:Article No. 123191.
Ebadollahzadeh H, Zabihi M. Competitive adsorption of methylene blue and Pb (II) ions on the nano-magnetic activated carbon and alumina. Materials Chemistry and Physics 2020;248:Article No. 122893.
Fang Q, Ye S, Yang H, Yang K, Zhou J, Gao Y, et al. Application of layered double hydroxide-biochar composites in wastewater treatment: Recent trends, modification strategies, and outlook. Journal of Hazardous Materials 2021;420:Article No. 126569.
Hu X, Li P, Zhang X, Yu B, Lv C, Zeng N, et al. Ni-based catalyst derived from NiAl layered double hydroxide for vapor phase catalytic exchange between hydrogen and water. Nanomaterials 2019;9(12):Article No. 1688.
Juleanti N, Palapa NR, Taher T, Hidayati N, Putri BI, Lesbani A. The capability of biochar-based CaAl and MgAl composite materials as adsorbent for removal Cr (VI) in aqueous solution. Science and Technology Indonesia 2021;6(3):156-65.
Karagöz S, Tay T, Ucar S, Erdem M. Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresource Technology 2008; 99(14):6214-22.
Khuluk RH, Rahmat A, Buhani, Suharso. Removal of methylene blue by adsorption onto activated carbon from coconut shell (Cocous nucifera L.). Indonesian Journal of Science and Technology 2019;4(2):229-40.
Lesbani A, Asri F, Palapa NR, Taher T, Rachmat A. Efficient removal of methylene blue by adsorption using composite based Ca/Al layered double hydroxide-biochar. Global NEST Journal 2020;22(2):250-7.
Li J, Zhao P, Li T, Lei M, Yan W, Ge S. Pyrolysis behavior of hydrochar from hydrothermal carbonization of pinewood sawdust. Journal of Analytical and Applied Pyrolysis 2020c;146(12):Article No. 104771.
Li Y, Peng L, Li W. Adsorption behaviors on trace Pb2+ from water of biochar adsorbents from konjac starch. Adsorption Science and Technology 2020b;38(9-10):344-56.
Li Z, Sellaoui L, Gueddida S, Dotto GL, Ben Lamine A, Bonilla-Petriciolet A, et al. Adsorption of methylene blue on silica nanoparticles: Modelling analysis of the adsorption mechanism via a double layer model. Journal of Molecular Liquids 2020a;319:Article No. 114348.
Liao W, Wang H, Li HQ, Yang P. Fe(II) Removal from aqueous solution by layered double hydroxide/graphene composites: Adsorption coupled with surface oxidation. Environmental Engineering Science 2020;37(1):43-52.
Lu Y, Chen J, Zhao L, Zhou Z, Qiu C, Li Q. Adsorption of rhodamine b from aqueous solution by goat manure biochar: Kinetics, isotherms, and thermodynamic studies. Polish Journal of Environmental Studies 2020;29(4):2721-30.
Mahmuda KN, Wen TH, Zakaria ZA. Activated carbon and biochar from pineapple waste biomass for the removal ofmethylene blue. Environmental and Toxicology Management 2021;1(1):30-6.
Mantasha I, Saleh HAM, Qasem KMA, Shahid M, Mehtab M, Ahmad M. Efficient and selective adsorption and separation of methylene blue (MB) from mixture of dyes in aqueous environment employing a Cu(II) based metal organic framework. Inorganica Chimica Acta 2020;511:Article No. 119787.
Mishra S, Sahoo SS, Debnath AK, Muthe KP, Das N, Parhi P. Cobalt ferrite nanoparticles prepared by microwave hydrothermal synthesis and adsorption efficiency for organic dyes: Isotherms, thermodynamics and kinetic studies. Advanced Powder Technology 2020;31(11):4552-62.
Nakhli A, Bergaoui M, Toumi KH, Khalfaoui M, Benguerba Y, Balsamo M, et al. Molecular insights through computational modeling of methylene blue adsorption onto low-cost adsorbents derived from natural materials: A multi-model’s approach. Computers and Chemical Engineering 2020; 140:Article No. 106965.
Normah N, Juleanti N, Siregar PMSBN, Wijaya A, Palapa NR, Taher T, et al. Size selectivity of anionic and cationic dyes using LDH modified adsorbent with low-cost rambutan peel to hydrochar. Bulletin of Chemical Reaction Engineering and Catalysis 2021a;16(4):869-80.
Normah, Palapa NR, Taher T, Mohadi R, Utami HP, Lesbani A. The ability of composite Ni/Al-carbon based material toward readsorption of iron(II) in aqueous solution. Science and Technology Indonesia 2021b;6(3):156-65.
Oliveira EIS, Santos JB, Gonçalves APB, Mattedi S, José NM. Characterization of the rambutan peel fiber (Nephelium lappaceum) as a lignocellulosic material for technological applications. Chemical Engineering Transactions 2016; 50:391-6.
Palapa NR, Taher T, Mohadi R, Rachmat A, Lesbani A. Preparation of copper aluminum-biochar composite as adsorbent of malachite green in aqueous solution. Research Square 2020a;524:1-24.
Palapa NR, Taher T, Rahayu BR, Mohadi R, Rachmat A, Lesbani A. CuAl LDH/Rice husk biochar composite for enhanced adsorptive removal of cationic dye from aqueous solution. Bulletin of Chemical Reaction Engineering and Catalysis 2020b;15(2):525-37.
Rafatullah M, Sulaiman O, Hashim R, Ahmad A. Adsorption of methylene blue on low-cost adsorbents: A review. Journal of Hazardous Materials 2010;177(1-3):70-80.
Rathee G, Awasthi A, Sood D, Tomar R, Tomar V, Chandra R. A new biocompatible ternary layered double hydroxide adsorbent for ultrafast removal of anionic organic dyes. Scientific Reports 2019;9(1):Article No. 16225.
Setiawan IKA, Napitupulu M, Walanda DK. Biocharcoal dari Kulit Rambutan (Nephelium lappaceum L.) sebagai Adsorben Zink dan Tembaga. Jurnal Akademika Kimia 2018; 7(4):Article No. 193.
Shin J, Kim K, Hong J. Zn-Al layered double hydroxide thin film. Coating 2020;10(669):1-7.
Stjepanović M, Velić N, Galić A, Kosović I, Jakovljević T, Habuda-Stanić M. From waste to biosorbent: Removal of congo red from water by waste wood biomass. Water 2021:13(3):Article No. 279.
Wang S, Gao B, Li Y, Zimmerman AR, Cao X. Sorption of arsenic onto Ni/Fe layered double hydroxide (LDH)-biochar composites. RSC Advances 2016;6:17792-9.
Wang W, Zhang N, Shi Z, Ye Z, Gao Q, Zhi M, et al. Preparation of Ni-Al layered double hydroxide hollow microspheres for supercapacitor electrode. Chemical Engineering Journal 2018;338:55-61.
Wang Z, Zhang L, Fang P, Wang L, Wang W. Study on simultaneous removal of dye and heavy metal Ions by NiAl-layered double hydroxide films. ACS Omega 2020; 5(34):21805-14.
Wijaya A, Siregar PNBSM, Priambodo A, Palapa NR, Taher T, Lesbani A. Innovative modified of Cu-Al/C (C=Biochar, Graphite) composites for removal of procion red from aqueous solution. Science Technology Indonesia 2021;6(4):228-34.
Wu J, Xia A, Chen C, Feng L, Su X, Wang X. Adsorption thermodynamics and dynamics of three typical dyes onto bio-adsorbent spent substrate of Pleurotus eryngii. International Journal of Environmental Research and Public Health 2019;16(5):Article No. 679.
Xu H, Zhang P, Zhou SY, Jia Q. Fullerene functionalized magnetic molecularly imprinted polymer: Synthesis, characterization and application for efficient adsorption of methylene blue. Chinese Journal of Analytical Chemistry 2020;48(9):e20107-e20113.
Xu H, Zhu S, Xia M, Wang F. Rapid and efficient removal of diclofenac sodium from aqueous solution via ternary core-shell CS@PANI@LDH composite: Experimental and adsorption mechanism study. Journal of Hazardous Materials 2021;402:Article No. 123815.
Yeow PK, Wong SW, Hadibarata T. Removal of azo and anthraquinone dye by plant biomass as adsorbent: A review. Biointerface Research in Applied Chemistry 2021;11(1): 8218-32.
Zhao J, Huang Q, Liu M, Dai Y, Chen J, Huang H, et al. Synthesis of functionalized MgAl-layered double hydroxides via modified mussel inspired chemistry and their application in organic dye adsorption. Journal of Colloid and Interface Science 2017;505:168-77.
Zhao Y, Zhan L, Xue Z, Yusef KK, Hu H, Wu M. Adsorption of Cu (II) and Cd (II) from wastewater by sodium alginate modified materials. Journal of Chemistry 2020;2020:Article No. 5496712.
Zubair M, Jarrah N, Ihsanullah KA, Manzar MS, Kazeem TS, Al-Harthi MA. Starch-NiFe-layered double hydroxide composites: Efficient removal of methyl orange from aqueous phase. Journal of Molecular Liquids 2018;249:254-64.