Selectivity of Malachite Green on Cationic Dye Mixtures Toward Adsorption on Magnetite Humic Acid 10.32526/ennrj/20/202200142
Main Article Content
Abstract
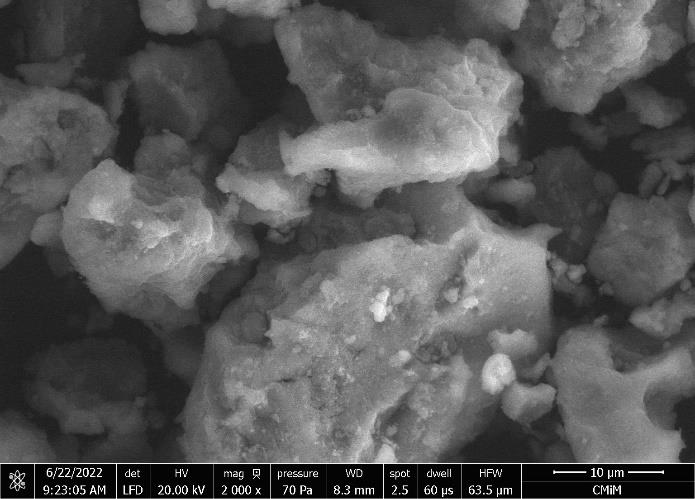
Magnetite humic acid (MHA) was successfully synthesized by the coprecipitation method followed by hydrothermal process, as evidenced by the XRD, FTIR, VSM, and SEM analysis characterization results. XRD diffraction shows diffraction peaks at 2=21.53º, 35.95º, and 57.93º. The FTIR spectra have a typical absorption at 3,410, 1,589, 1,396, 1,026, 910, 794, and 540 cm-1. Magnetite humic acid was paramagnetic with magnetization (Ms) 17.04 emu/g. Humic acid and magnetite humic acid have an irregular structure; the morphology of magnetite humic acid is smoother than humic acid. Malachite green was more selective than methylene blue and rhodamine B on magnetite humic acid. The adsorption of malachite green on humic acid and magnetite humic acid was carried out at pHpzc 8.06 and 6.08. The adsorption capacity (Qmax) of humic acid (77.519 mg/g) and magnetite humic acid (169.492 mg/g) were found with pseudo-second-order kinetic and Langmuir isotherm models. After five regeneration cycles, the adsorption percentages of malachite green with humic acid and magnetite humic acid ranged from 94.67-61.37% and 62.03-21.11%, respectively. Magnetite humic acid has high stability and reusability. The good regeneration of MHA was supported by the XRD diffractogram. Magnetic properties in the material simplify the adsorption process and minimize the potential for damage to the surface of the material.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Abdullah TA, Juzsakova T, Mansoor H, Salman AD, Rasheed RT, Hafad SA, et al. Polyethylene over magnetite-multiwalled carbon nanotubes for kerosene removal from water. Chemosphere 2022;287:Article No. 132310.
Ahmad N, Wijaya A, Amri, Fitri ES, Arsyad FS, Mohadi R, et al. Catalytic oxidative desulfurization of dibenzothiophene by composites based Ni/Al-Oxide. Science and Technology Indonesia 2022;7(3):385-91.
Al-Yousef HA, Alotaibi BM, Alanazi MM, Aouaini F, Sellaoui L, Bonilla-Petriciolet A. Theoretical assessment of the adsorption mechanism of ibuprofen, ampicillin, orange G and malachite green on a biomass functionalized with plasma. Journal of Environmental Chemical Engineering 2021;9(1):Article No. 104950.
Angerasa FT, Kalifa MA, Jembere AL, Genet MB. Spent kaolin filter cake as an effective adsorbent for the removal of hexavalent chromium [Cr(VI)] from aqueous solution: Comparative study of wastewater treatment methods. South African Journal of Chemical Engineering 2021;38:90-103.
Anjum H, Johari K, Gnanasundaram N, Appusamy A, Thanabalan M. Investigation of green functionalization of multiwall carbon nanotubes and its application in adsorption of benzene, toluene, and p-xylene from aqueous solution. Journal of Cleaner Production 2019;221:323-38.
Chen H, Liu T, Meng Y, Cheng Y, Lu J, Wang H. Novel graphene oxide/aminated lignin aerogels for enhanced adsorption of malachite green in wastewater. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2020;603:Article No. 125281.
Das KC, Dhar SS. Rapid catalytic degradation of malachite green by MgFe2O4 nanoparticles in presence of H2O2. Journal of Alloys and Compounds 2020;828:Article No. 154462.
Derakhshani E, Naghizadeh A. Optimization of humic acid removal by adsorption onto bentonite and montmorillonite nanoparticles. Journal of Molecular Liquids 2018;259:76-81.
Eltaweil AS, Mohamed HA, El-Monaem EMA, El-Subruiti GM. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Advanced Powder Technology 2020;31(3):1253-63.
Fazal T, Mushtaq A, Rehman F, Khan AU, Rashid N, Farooq W, et al. Bioremediation of textile wastewater and successive biodiesel production using microalgae. Renewable and Sustainable Energy Reviews 2018;82:3107-26.
Giri BS, Sonwani RK, Varjani S, Chaurasia D, Varadavenkatesan T, Chaturvedi P, et al. Highly efficient bio-adsorption of malachite green using Chinese Fan-Palm Biochar (Livistona chinensis). Chemosphere 2022;287:Article No. 132282.
Hijab M, Parthasarathy P, Mackey HR, Al-Ansari T, McKay G. Minimizing adsorbent requirements using multi-stage batch adsorption for malachite green removal using microwave date-stone activated carbons. Chemical Engineering and Processing - Process Intensification 2021;167:Article No. 108318.
Januário EFD, Vidovix TB, Calsavara MA, Bergamasco R, Vieira AMS. Membrane surface functionalization by the deposition of polyvinyl alcohol and graphene oxide for dyes removal and treatment of a simulated wastewater. Chemical Engineering and Processing - Process Intensification 2022;170:Article No. 108725.
Khalaf MA. Biosorption of reactive dye from textile wastewater by non-viable biomass of Aspergillus niger and Spirogyra sp. Bioresource Technology 2008;99(14):6631-4.
Koesnarpadi S, Santosa SJ, Siswanta D, Rusdiarso B. Synthesis and characterizatation of magnetite nanoparticle coated humic acid (Fe3O4/HA). Procedia Environmental Sciences 2015; 30:103-8.
Lee WH, Kim JO. Phosphate recovery from anaerobic digestion effluent using synthetic magnetite particles. Journal of Environmental Chemical Engineering 2022;10(1):Article No. 107103.
Lesbani A, Asri F, Palapa NR, Taher T, Rachmat A. Efficient removal of methylene blue by adsorption using composite based Ca/Al layered double hydroxide-biochar. Global Nest Journal 2020;22(2):250-7.
Liu Y, Jia J, Gao T, Wang X, Yu J, Wu D, et al. Rapid, selective adsorption of methylene blue from aqueous solution by durable nanofibrous membranes. Journal of Chemical and Engineering Data 2020;65(8):3998-4008.
Liu Y, Liu Q, Li J, Ngo HH, Guo W, Hu J, et al. Effect of magnetic powder on membrane fouling mitigation and microbial community/composition in membrane bioreactors (MBRs) for municipal wastewater treatment. Bioresource Technology 2018;249:377-85.
Lu M, Zhang Y, Zhou Y, Su Z, Liu B, Li G, et al. Adsorption-desorption characteristics and mechanisms of Pb(II) on natural vanadium, titanium-bearing magnetite-humic acid magnetic adsorbent. Powder Technology 2019;344:947-58.
Miyar HK, Pai A, Goveas LC. Adsorption of malachite green by extracellular polymeric substance of Lysinibacillus sp. SS1: Kinetics and isotherms. Heliyon 2021;7(6):e07169.
Mohadi R, Palapa NR, Lesbani A. Preparation of Ca/Al-layered double hydroxides/biochar composite with high adsorption capacity and selectivity toward cationic dyes in aqueous. Bulletin of Chemical Reaction Engineering and Catalysis 2021;16(2):244-52.
Palapa NR, Mohadi R, Rachmat A, Lesbani A. Adsorption study of malachite green removal from aqueous solution using Cu/M3+ (M3+=Al, Cr) layered double hydroxide. Mediterranean Journal of Chemistry 2020;10(1):33-45.
Palapa NR, Taher T, Wijaya A, Lesbani A. Modification of Cu/Cr layered double hydroxide by keggin type polyoxometalate as adsorbent of malachite green from aqueous solution. Science and Technology Indonesia 2021;6(3):209-17.
Paz MJ, Vieira T, Enzweiler H, Paulino AT. Chitosan/wood sawdust/magnetite composite membranes for the photo-degradation of agrochemicals in water. Journal of Environmental Chemical Engineering 2022;10(1):Article No. 106967.
Rashid M, Price NT, Pinilla MÁG, O’Shea KE. Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles. Water Research 2017;123(3):353-60.
Roohi M, Riaz M, Arif MS, Shahzad SM, Yasmeen T, Riaz MA, et al. Varied effects of untreated textile wastewater onto soil carbon mineralization and associated biochemical properties of a dryland agricultural soil. Journal of Environmental Management 2016;183:530-40.
Sachdev D, Shrivastava H, Kajal, Sharma S, Sanjeev, Srivastava S, et al. Potential for hydrothermally separated groundnut shell fibers for removal of methylene blue dye. Materials Today: Proceedings 2022;48:1559-68.
Sadiq AC, Rahim NY, Suah FBM. Adsorption and desorption of malachite green by using chitosan-deep eutectic solvents beads. International Journal of Biological Macromolecules 2020;164:3965-73.
Santosa SJ, Krisbiantoro PA, Ha TTM, Phuong NTT, Gusrizal G. Composite of magnetite and Zn/Al layered double hydroxide as a magnetically separable adsorbent for effective removal of humic acid. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021b;614:Article No. 126159.
Santosa SJ, Krisbiantoro PA, Yuniarti M, Kustomo, Koesnarpardi S. Magnetically separable humic acid-functionalized magnetite for reductive adsorption of tetrachloroaurate(III) ion in aqueous solution. Environmental Nanotechnology, Monitoring and Management 2021a;15:Article No. 100454.
Shao Y, Bao M, Huo W, Ye R, Liu Y, Lu W. Production of artificial humic acid from biomass residues by a non-catalytic hydrothermal process. Journal of Cleaner Production 2021;335:Article No. 130302.
She HD, Fan HR, Yang KF, Li XC, Wang QW, Zhang LF, et al. In situ trace elements of magnetite in the Bayan Obo REE-Nb-Fe deposit: Implications for the genesis of mesoproterozoic iron mineralization. Ore Geology Reviews 2021;139:Article No. 104574.
Shen X, Dong W, Wan Y, Feng K, Liu Y, Wei Y. Influencing mechanisms of siderite and magnetite, on naphthalene biodegradation: Insights from degradability and mineral surface structure. Journal of Environmental Management 2021;299:Article No. 113648.
Signorelli SCM, Costa JM, Neto AFA. Electrocoagulation-flotation for orange II dye removal: Kinetics, costs, and process variables effects. Journal of Environmental Chemical Engineering 2021;9(5):Article No. 106157.
Siraorarnroj S, Kaewtrakulchai N, Fuji M, Eiad-ua A. High performance nanoporous carbon from mulberry leaves (Morus alba L.) residues via microwave treatment assisted hydrothermal-carbonization for methyl orange adsorption: Kinetic, equilibrium and thermodynamic studies. Materialia 2022;21:Article No. 101288.
Srivastava S, Sinha R, Roy D. Toxicological effects of malachite green. Aquatic Toxicology 2004;66(3):319-29.
Stevenson FJ. Humus Chemistry: Genesis, Composition, Reactions. USA: John Wiley and Sons; 1994.
Taher T, Putra R, Palapa NR, Lesbani A. Preparation of magnetite-nanoparticle-decorated NiFe layered double hydroxide and its adsorption performance for congo red dye removal. Chemical Physics Letters 2021;777:Article No. 138712.
Tombácz E, Szekeres M. Colloidal behavior of aqueous montmorillonite suspensions: The specific role of pH in the presence of indifferent electrolytes. Applied Clay Science 2004;27(1-2):75-94.
Vigneshwaran S, Sirajudheen P, Karthikeyan P, Meenakshi S. Fabrication of sulfur-doped biochar derived from tapioca peel waste with superior adsorption performance for the removal of malachite green and rhodamine B dyes. Surfaces and Interfaces 2021;23:Article No. 100920.
Vu MT, Noori MT, Min B. Conductive magnetite nanoparticles trigger syntrophic methane production in single chamber microbial electrochemical systems. Bioresource Technology 2020;296:Article No. 122265.
Wang M, Li Y, Cui M, Li M, Xu W, Li L, et al. Barium alginate as a skeleton coating graphene oxide and bentonite-derived composites: Excellent adsorbent based on predictive design for the enhanced adsorption of methylene blue. Journal of Colloid and Interface Science 2021a;611:629-43.
Wang Y, Bao S, Liu Y, Yu Y, Yang W, Xu S, et al. CoS2/GO nanocomposites for highly efficient and ppb level adsorption of Hg(II) from wastewater. Journal of Molecular Liquids 2021b;322:Article No. 114899.
Wijaya A, Siregar PMSBN, Priambodo A, Palapa NR, Taher T, Lesbani A. Innovative modified of Cu-Al/C (C=biochar, graphite) composites for removal of procion red from aqueous solution. Science and Technology Indonesia 2021;6(4):228-34.
Yang F, Antonietti M. Artificial humic acids: Sustainable materials against climate change. Advanced Science 2020;7(5):1-7.
You J, Liu C, Feng X, Lu B, Xia L, Zhuang X. In situ synthesis of ZnS nanoparticles onto cellulose/chitosan sponge for adsorption-photocatalytic removal of congo red. Carbohydrate Polymers 2022;288:Article No. 119332.
Zhang A, Chen W, Gu Z, Li Q, Shi G. Mechanism of adsorption of humic acid by modified aged refuse. RSC Advantages 2018;8:33642-51.
Zhang T, Jin X, Owens G, Chen Z. Remediation of malachite green in wastewater by ZIF-8@Fe/Ni nanoparticles based on adsorption and reduction. Journal of Colloid and Interface Science 2021;594:398-408.
Zhang W, Zhang RZ, Yin Y, Yang JM. Superior selective adsorption of anionic organic dyes by MIL-101 analogs: Regulation of adsorption driving forces by free amino groups in pore channels. Journal of Molecular Liquids 2020; 302:Article No. 112616.