Effect of Oxide Presence in Activated Carbon on Arsenic Removal 10.32526/ennrj/21/20230066
Main Article Content
Abstract
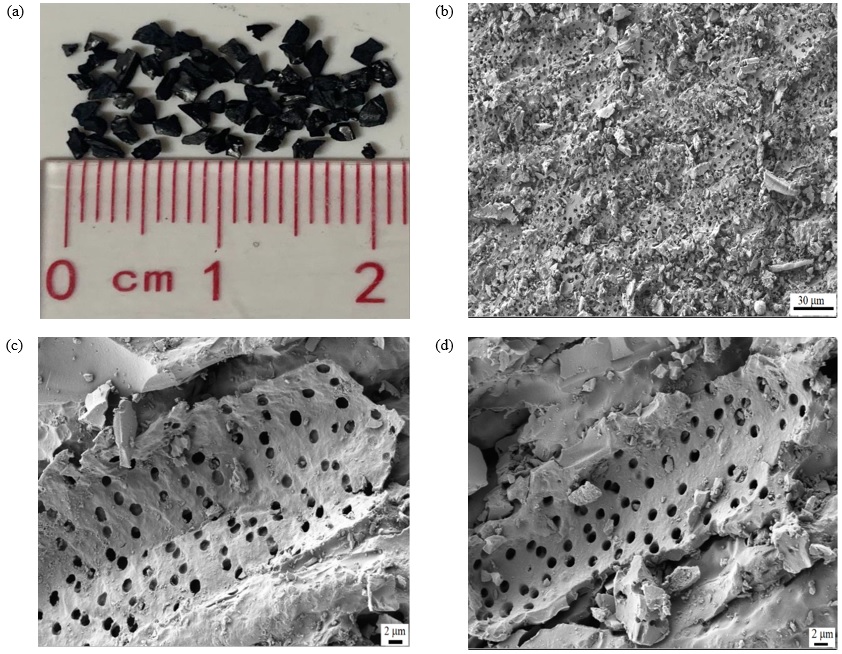
This study investigated the effect of oxides on the removal of As when present in simple mixtures with granular activated carbon (GAC) particles. The performance of these mixtures was compared with other reported GAC-based adsorbents. A standard curve for ultraviolet adsorption vs. As concentration was obtained using the silver diethyldithiocarbamate (SDDC) method to evaluate various samples. A preliminary study was carried out to find the optimal conditions for experiments. For 50 mL samples with 2.35 ppm As, the optimal values of pH, adsorption time, and amount of adsorbent were pH 7, 30 min, and 50 mg, respectively. The ratio between the amount of adsorbent and well water in this study showed a superior As adsorption capacity (1 g/L, 2.1 mg/g) compared to similar adsorbents reported previously (12.5 g/L, 1.0-1.4 mg/g). Among the adsorbents, KOH-treated AC-Mn3O4 exhibited the best performance in As removal with an efficiency of ~95%. The oxide particles had a synergistic effect with GAC on As removal. This was primarily due to the change in the potential of partially agglomerated nano Mn3O4 particles on the ACK surface. The influence of the surface area of the adsorbents was not pronounced. All results were explained in terms of microstructure, specific surface area, and zeta potential. This finding could be extended to other activated carbons (AC) obtained from different sources.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: Implications and challenges for healthcare policy. Risk Management and Healthcare Policy 2018;11:251-61.
Chang M, Shih YH. Synthesis and application of magnetic iron oxide nanoparticles on the removal of Reactive Black 5: Reaction mechanism, temperature and pH effects. Journal of Environmental Management 2018;224:235-42.
Dehmani Y, Abouarnadasse S. Study of the adsorbent properties of nickel oxide for phenol depollution. Arabian Journal of Chemistry 2020;13(5):5312-25.
Dukhin AS, Goetz PJ. Characterization of Liquids, Dispersions, Emulsions, and Porous Materials Using Ultrasound. 3rd ed. Elsevier; 2017.
Esmaeili H, Mousavi SM, Hashemi SA, Chiang WH, Abnavi SA. Activated carbon@ MgO@ Fe3O4 as an efficient adsorbent for As (III) removal. Carbon Letters 2021;31:851-62.
Gil A, Galeano LA, Vicente MÁ. Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment. Cham: Springer International Publishing; 2019.
Hudak PF. Nitrate, arsenic and selenium concentrations in the pecos valley aquifer, West Texas, USA. International Journal of Environmental Research 2010;4(2):229-36.
Jha VK, Maharjan J. Activated carbon obtained from banana peels for the removal of AS (III) from water. Scientific World 2022;15(15):145-57.
Jiang W, Zhang L, Guo X, Yang M, Lu Y, Wang Y, et al. Adsorption of cationic dye from water using an iron oxide/activated carbon magnetic composites prepared from sugarcane bagasse by microwave method. Environmental Technology 2021;42(3):337-50.
Joshi S, Sharma M, Kumari A, Shrestha S, Shrestha B. Arsenic removal from water by adsorption onto iron oxide/nano-porous carbon magnetic composite. Applied Sciences 2019;9(18):Article No. 3732.
Kalaruban M, Loganathan P, Nguyen TV, Nur T, Johir MA, Nguyen TH, et al. Iron-impregnated granular activated carbon for arsenic removal: Application to practical column filters. Journal of Environmental Management 2019;239:235-43.
Koohzad E, Jafari D, Esmaeili H. Adsorption of lead and arsenic ions from aqueous solution by activated carbon prepared from tamarix leaves. ChemistrySelect 2019;4(42):12356-67.
Kumar A, Dixit CK. Methods for characterization of nanoparticles. In: Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids. United Kingdom: Woodhead Publishing; 2017.
Liang M, Lai Y. Determination of the arsenic content in surface water by silver diethyldithiocarbamate spectrophotometry. Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering; 2010 Jun 18-20; Chengdu: China; 2010.
López-Guzmán M, Alarcón-Herrera MT, Irigoyen-Campuzano JR, Torres-Castañón LA, Reynoso-Cuevas L. Simultaneous removal of fluoride and arsenic from well water by electrocoagulation. Science of the Total Environment 2019; 678:181-7.
Mahmoodi NM, Ghezelbash M, Shabanian M, Aryanasab F, Saeb MR. Efficient removal of cationic dyes from colored wastewaters by dithiocarbamate-functionalized graphene oxide nanosheets: From synthesis to detailed kinetics studies. Journal of the Taiwan Institute of Chemical Engineers 2017;81:239-46.
Mahmoodi NM. Synthesis of magnetic carbon nanotube and photocatalytic dye degradation ability. Environmental Monitoring and Assessment 2014;186(9):5595-604.
Mostafapour FK, Bazrafshan E, Farzadkia M, Amini S. Arsenic removal from aqueous solutions by Salvadora persica stem ash. Journal of Chemistry 2013;2013:Article No. 740847.
Mousavi SR, Asghari M, Mahmoodi NM. Chitosan-wrapped multiwalled carbon nanotube as filler within PEBA thin film nanocomposite (TFN) membrane to improve dye removal. Carbohydrate Polymers 2020;237:Article No. 116128.
Pravalprukskul P, Aung MT, Wichelns D. Arsenic in Rice: State of Knowledge and Perceptions in Cambodia. Stockholm: Stockholm Environment Institute; 2018.
Rahaman MN. Ceramic Processing and Sintering. Boca Raton: CRC Press; 2017.
Rahman HL, Erdem H, Sahin M, Erdem M. Iron-incorporated activated carbon synthesis from biomass mixture for enhanced arsenic adsorption. Water, Air, and Soil Pollution 2020;231:1-7.
Rusmana YF, Notodarmojo S, Helmy Q. Arsenic removal in groundwater by integrated ozonation and adsorption by activated carbon and zeolite. IOP Conference Series: Materials Science and Engineering 2019;536(1):Article No. 012073.
Stratton G, Whitehead HC. Colorimetric determination of arsenic in water with silver diethyldithiocarbamate. Journal‐American Water Works Association 1962;54(7):861-4.
Tallman DE, Shaikh AU. Redox stability of inorganic arsenic (III) and arsenic (V) in aqueous solution. Analytical Chemistry 1980;52(1):196-9.
Tan IA, Ahmad AL, Hameed BH. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. Journal of Hazardous Materials 2008;154(1-3):337-46.
Vašák V, Šedivec V. Colorimetric determination of arsenic. Collection of Czechoslovak Chemical Communications 1953;18(1):64-72.
Thearak V. Oxides and Activated Carbon on Removal of Arsenic from Cambodian Well Water [dissertation]. Phnom Penh, Royal University of Phnom Penh; 2023.
World Health Organization (WHO). Hardness in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. WHO; 2010.
World Health Organization (WHO). Guidelines for Drinking-Water Quality: WHO Chronicle. Switzerland: WHO; 2011.
Wong S, Ngadi N, Inuwa IM, Hassan O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. Journal of Cleaner Production 2018;175:361-75.
Yao S, Liu Z, Shi Z. Arsenic removal from aqueous solutions by adsorption onto iron oxide/activated carbon magnetic composite. Journal of Environmental Health Science and Engineering 2014;12:1-8.