Seed Osmopriming Improves Germination, Physiological, and Root Anatomical Attributes of Red Amaranth (Amaranthus tricolor L.) in Salinity Stress Condition 10.32526/ennrj/21/202200258
Main Article Content
Abstract
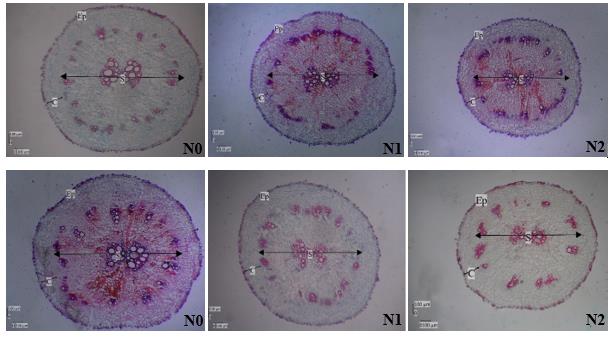
Salinity stress is a form of abiotic stress that threatens the sustainability of agriculture in almost all countries in the world. It has an impact in reducing plant productivity. Red amaranth (Amaranthus tricolor L.) is a vegetable crop that has high nutritional value, but extensive saline land area can cause red amaranth yields to decline. Osmopriming is a seed priming method in which seeds are immersed in a solution that has a high osmotic potential, such as PEG (polyethylene glycol) in order to increase germination under unfavorable conditions. This study determined the effect of osmopriming on germination, physiological, and root anatomical attributes of red amaranth roots under salinity stress conditions. The research design used a completely randomized design with two types of treatment, namely, osmopriming and salinity stress. Each treatment used three concentrations, seed osmopriming with 0%, 5%, and 10% of PEG and salinity stress of 0 mM, 50 mM, and 100 mM of NaCl. The measured parameters were germination, growth, physiological, and root anatomical characters. Osmopriming of seeds with 10% PEG increased germination as indicated by the germination percentage, time, and rate reaching 95.55%, 1.393 day, and 71.98%/day, respectively. Red amaranth plants that had been osmoprimed with 10% PEG grew faster when exposed to salinity stress. Application of PEG 5% and 10% increased total chlorophyll levels while decreasing proline levels and Ca-oxalate crystal density. Under salinity stress conditions, PEG application improved the root anatomical characters of red amaranth as shown by increased epidermis thickness, cortex thickness, and stele diameter. Priming application with 10% PEG has the potential to increase the tolerance of red amaranth to salinity stress.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Abdelaziz MN, Xuan TD, Mekawy AMM, Wang H, Khanh TD. Relationship of salinity tolerance to Na+ exclusion, proline accumulation, and antioxidant enzyme activity in rice seedlings. Agriculture 2018;8(166):1-12.
Abdelhamid MT, El-Masry RR, Saleh DD, Mazhar MF, Ragab R, Oba S, et al. Mechanisms of seed priming involved in salt stress amelioration. In: Hasanuzzaman M, Fotopoulos V, editors. Priming and Pretreatment of Seeds and Seedlings Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants. Singapore: Springer; 2019. p. 219-51.
Abid M, Hakeem A, Shao Y, Liu Y, Zahoor R, Fan Y, et al. Seed osmopriming invokes stress memory against post-germinative drought stress in wheat (Triticum aestivum L.). Environmental and Experimental Botany 2017;145:12-20.
Abid M, Zhang YJ, Li Z, Bai DF, Zhong YP, Fang JB. Effect of Salt stress on growth, physiological and biochemical characters of four kiwi fruit genotypes. Scientia Horticulturae 2020;271:1-12.
Ahmad P, Azooz MM, Prasad MNV. Ecophysiology and Responses of Plants under Salt Stress. New York: Springer; 2013. p. 16.
Al-Mudaris MA. Notes on various parameters recording the speed of seed germination. Der Tropenlandwirt 1998;99:147-54.
Alleva K, Chara O, Amodeo G. Aquaporins: Another piece in the osmotic puzzle. FEBS Letters 2012;586:2991-9.
Alzahrani SM, Alaraidh IA, Migdadi H, Alghamdi S, Altaf KM, Ahmad P. Physiological, biochemical, and antioxidant properties of two genotypes of Vicia faba grown under salinity stress. Pakistan Journal of Botany 2019;51:786-98.
Amalia DR. The Effect of Seed Osmopriming with PEG 6000 (Polyethylene Glycol) on Growth and Morphophysiological of Red Amaranth (Amaranthus tricolor L.) in NaCl Stress [dissertation]. Faculty of Biology, Universitas Gadjah Mada; 2022.
Arjmand HS, Sharafi S, Jouyban Z, Akhlaghi S. Effects of osmopriming on seed germination, emergence and field performance. Journal of Applied Science and Agriculture 2014;9(4):1569-73.
Atabayeva S, Nurmahanova A, Minocha S, Ahmetova A, Kenzhebayeva S, Aidosova S, et al. The effect of salinity on growth and anatomical attributes of Barley seedling (Hordeum vulgare L.). African Journal of Biotechnology 2013; 12(18):2366-77.
Bates LS, Waldran RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil 1973;39:Article No. 205.
Battaglia M, Covarrubias AA. Late embryogenesis abundant (LEA) proteins in legumes. Frontiers in Plant Science 2014;4(190):1-11.
Benincasa P, Pace R, Quinet M, Lutts S. Effect of salinity and priming on seedling growth in Rapeseed (Brassica napus var oleifera Del.). Acta Scientiarum Agronomy 2013;35(4):479-86.
Boughalleb F, Denden M, Tiba B. Anatomical changes induced by increasing NaCl salinity in three fodder shrubs, Nitraria retusa, Atriplex halimus, and Medicago arborea. Acta Physiologiae Plantarum 2009;31:947-60.
Boughalleb F, Abdellaoui R, Nbiba N, Mahmoudi M, Neffati M. Effect of NaCl stress on physiological, antioxidant enzymes and anatomical responses of Astragalus gombiformis. Biologia 2017;72(12):1454-66.
Cai X, Ge C, Xu C, Wang X, Wang S, Wang Q. Expression analysis of oxalate metabolic pathway genes reveals oxalate regulation patterns in Spinach. Molecules 2018;23(6):Article No. 1286.
Chaudhry AH, Nayab S, Hussain SB, Ali M, Pan Z. Current understandings on magnesium deficiency and future outlooks for sustainable agriculture. International Journal of Molecular Sciences 2021;22(4):1-18.
Chen X, Zhang R, Xing Y, Jiang B, Li B, Xu X. The efficacy of different seed osmopriming agents for promoting sorghum germination under salt stress. PLoS ONE 2021;16(1):1-14.
da Silva JG, Bianchini A, Costa PMC, de Almeida Lobo F, de Ameida JPM, de Moraes MF. Amaranth response to water stress. Journal of Experimental Agricultural International 2019;40(1):1-9.
Das K, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science 2014;2(53):1-13.
Debbarma M, Das SP. Priming of seed: Enhancing growth and development. International Journal of Current Microbiology and Applied Sciences 2017;6(12):2390-6.
Fang S, Hou X, Liang X. Response mechanisms of plants under saline-alkali stress. Frontiers in Plant Science 2021; 12(667458):1-20.
Farooq M, Usman M, Nadeem F. Seed priming in field crops: Potential benefits, adoption and challenges. Crop and Pasture Science 2019;70:731-71.
Fitriani H, Nurlailah D, Rakhimna. Oxalic acid content in spinach. Medical Laboratory Technology Journal 2016;2(2):51-5. (in Indonesian).
Fort F, Jouany C, Cruz P. Root and leaf functional trait relations in Poaceae species: Implications of differing resource-acquisition strategies. Journal of Plant Ecology 2013; 6(3):211-9.
Ghiyasi M, Tajbakhsh M. Osmopriming alleviates drought stress in Soybean (Glycine max L.) seeds during germination and early growth stages. Journal of Applied Biological Sciences 2013;7(1):27-32.
Harijati N, Arumingtyas EL, Handayani R. The effect of calcium on the size and density of crystal of calcium oxalate in Porang (Amorphophallus muelleri Blume). Jurnal Pembangunan dan Alam Lestari 2011;1:72-139. (in Indonesian)
Hasan R, Miyake H. Salinity stress alters nutrient uptake and causes the damage of root and leaf anatomy in maize. KnE Life Sciences 2017;3(4):219-25.
Hasanuzzaman M, Parvin K, Anee TI, Masud AAC, Nowroz F. Salt stress responses and tolerance in soybean. In: Hasanuzzaman M, Nahar K, editors. Plant Stress Physiology-Perspectives in Agriculture. IntechOpen; 2022. p. 1-37.
Hoang HL, Guzman C, Cadiz N, Dang HT. Physiological and phytochemical responses of red amaranth (Amaranthus tricolor L.) and green amaranth (Amaranthus dubius L.) to different salinity levels. Agricultural Research Com-munication Centre 2019;2(9):1-7.
Hussian I, Ahmad R, Farooq M, Rehman A, Amin M, Bakar MA. Seed priming: A tool to invigorate the seeds. Scientia Agriculturae 2014;7(3):122-8.
Kaur S, Gupta AK, Kaur N. Seed priming increase crop yield possibly by modulating enzymes of sucrose metabolism in chickpea. Journal of Agronomy and Crop Science 2005;191:81-7.
Khan AH, Singh AK, Maurya KN, Yadava RK. Effect of different seed priming treatments on germination, growth, biochemical changes, and yield of wheat varieties under sodic soil. International Journal of Science and Research 2015; 4(7):Article No. 14061502 .
Kibria MG, Hoque Md A. A Review on plant responses to soil salinity and amelioration strategies. Open Journal of Soil Science 2019;9:219-31.
Kim SH, Su YL, Jae YH. The effect of seed priming on the germination properties of Aruncus dioicus. Seed Science and Technology 2022;50(2):221-6.
Kotagiri D, Kolluru VC. Effect of salinity stress on the morphology and physiology of five different coleus species. Biomedical and Pharmacology Journal 2017;10(4):1639-49.
Kubala S, Garnczarska M, Wojtyla Ł, Clippe A, Kosmala A, Żmieńko A, Quinet M. Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Science 2015;231:94-113.
Latifa A, Rachmawati D. The effect of seed osmopriming on growth and morphophysiology of Kangkoong (Ipomoea reptans Poir) in drought stress. Jurnal Agronomi Indonesia 2020;48(2):165-72. (in Indonesian).
Lei C, Bagavathiannan M, Wang H, Sharpe S, Meng W, Yu J. Osmopriming with polyethylene glycol (PEG) for abiotic stress tolerance in germinating crop seeds: A Review. Agronomy 2021;11(11):Article No. 2194.
Li G, Meng X, Zhu M, Li Z. Research progress of betalain in response to adverse stresses and evolutionary relationship compared with anthocyanin. Molecules 2019;24(17):Article No. 3078.
Maksimov IV, Surina OB, Sakhabutdinova AR, Troshina NB, Shakirova FM. Changes in the phytohormone levels in wheat calli as affected by salicylic acid and infection with Tilletia caries, a bunt pathogenic agent. Plant Physiology 2004; 51:228-33.
Mane AV, Karadge BA, Samant JS. Salinity induced changes in photosynthetic pigments and polyphenols of Cymbopogon Nardus (L.) Rendle. Journal of Chemical and Pharmaeutical Research 2010;2:338-47.
Menezes RV, Azevedo Neto ADD, Ribeiro MDO, Cova AMW. Growth and contents of organic and inorganic solutes in amaranth under salt stress. Pesquisa Agropecuária Tropical 2017;47:22-30.
Morales SG, Trejo-Tellez LI, Merino FCG, Caldana C, Espinosa-Victoria D, Cabrera BEH. Growth, photosynthetic activity, and potassium and sodium concentration in rice plants under salt stress. Acta Scientiarum-Agronomy 2012;34(3):317-24.
Nakata P. Plant calcium oxalate crystal formation, function, and its impact on human health. Frontiers in Biology 2012; 7:254-66.
Nxele X, Klein A, Ndimba BK. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. South African Journal of Botany 2017; 108:261-6.
Omami EN, Hammes PS, Robbertse PJ. Differences in salinity tolerance for growth and water‐use efficiency in some amaranth (Amaranthus spp.) genotypes. New Zealand Journal of Crop and Horticultural Science 2006;34(1):11-22.
Pallaoro DS, Camili EC, Guimarães S, de Figueiredo e Albuquerque MC. Methods for priming maize seeds. Journal of Seed Science 2016;38(2):148-54.
Parera CA, Cantliffe DJ. Pre-sowing seed priming. Horticultural Reviews 1994;16:109-41.
Pospíšil P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Frontiers in Plant Science 2016;7:1-11.
Price CA, Wright IJ, Ackerly DD, Niinemets U, Reich PB, Veneklaas EJ. Are leaf functional traits ‘invariant’ with plant size and what is ‘invariance’ anyway? Functional Ecology 2014;28:1330-43.
Puvanitha S, Mahendran S. Effect of salinity on plant height, shoot and root dry weight of selected rice cultivars. Scholars Journal of Agriculture and Veterinary Sciences 2017;4(4):126-31.
Rachma TNS, Damanhuri, Saptadi D. Viability and vigor of Cacao (Theobroma cacao L.) in some different invigoration media. Plantropica Journal of Agricultural Science 2016;1(2):72-80. (in Indonesian).
Raj AB, Raj SK. Seed priming: An approach towards agricultural sustainability. Journal of Applied and Natural Science 2019;11:227-34.
Rehman A, Nadeem F, Farooq M. Role of seed priming in root development and crop production. In: Rengel Z, Djalovic I, editors. The Root Systems in Sustainable Agricultural Intensification. United States: John Wiley and Sons Ltd.; 2021. p. 221-5.
Rosawanti P, Ghulamahdi M, Khumaida N. Anatomical and physiological responses of soybean root to drought stress. Jurnal Agronomi Indonesia 2015;43(3):186-92. (in Indonesian).
Ruzin SE. Plant Microtechnique and Microscopy. New York: Oxford University Press; 1999.
Tooulakou G, Giannopoulos A, Nikolopoulos D, Bresta P, Dotsika E, Orkoula MG, et al. Alarm photosynthesis: Calcium oxalate crystals as an internal CO2 source in plants. Plant Physiology 2016;171:2577-85.
Uddin S, Nafees M. Effect of seed priming on growth and performance of Vigna radiata L. under induced drought stress. Journal of Agriculture and Food Research 2021;4:1-8.
Wang X, Geng S, Ri YJ, Cao D, Liu J, Shi D, et al. Physiological responses and adaptive strategies of tomato plants to salt and alkali stresses. Scientia Horticulturae 2011;130:248-55.
Wulandari Y, Triadiati T, Sulistyaningsih Y, Suprayogi A, Rahminiwati M. Salinity stress affects growth and physiology of mulberry (Morus sp.). IOP Conference Series: Earth and Environmental Science 2021;948:1-11.
Xu HW, Ji XM, He ZH, Shi WP, Zhu GH, Niu JK, et al. Oxalate accumulation and regulation is independent of glycolate oxidase in rice leaves. Journal of Experimental Botany 2006;57(9):1899-908.
Yildiz M, Poyraz I, Caudar A, Ozgen Y, Beyaz R. Plant response to salt stress. In: Abdurakhmonov IY, editor. Plant Breeding: Current and Future Views. IntechOpen; 2020. p. 1-19.
Yoshida S, Forno DA, Cock JH, Gomez KA. Laboratory Manual for Physiological Studies of Rice. 3rd ed. Philippines: The International Rice Research Institute. 1976.