Phenolated Alkali Lignin/Magnetite Composite as an Adsorbent for Methyl Violet 6B in Wastewater 10.32526/ennrj/22/20230256
Main Article Content
Abstract
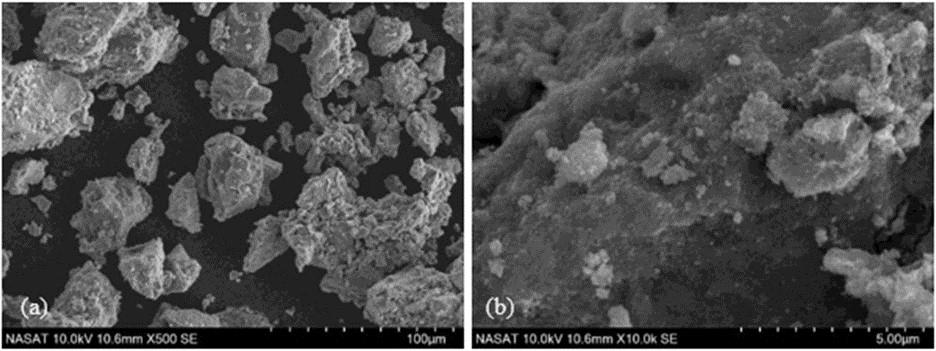
Methyl violet 6B (MV6B), found in wastewater, poses hazardous effects to aquatic ecosystems and human health; therefore, it must be removed immediately. In response, this study pioneered the development of a dye adsorbent by incorporating phenolated alkali lignin (PAL) into magnetite (Fe3O4), offering a solution for MV6B removal. Lignin was extracted from coconut husk through alkali extraction, chemically modified using phenolation, and integrated onto the magnetite surface. SEM and FTIR spectroscopy were used to characterize the adsorbent, and various parameters were optimized, along with evaluations of the adsorption kinetics and isotherm models, as well as the adsorbent’s reusability. PAL was successfully deposited onto the magnetite based on the characterization. The experimental results revealed that the optimal conditions for the removal of MV6B using PAL/Fe3O4 composite are pH 4, a temperature of 313 K, a dosage of 0.10 g PAL/Fe3O4 per 15 mL of MV6B, and a contact time of 150 minutes. MV6B’s equilibrium removal rate was 95.1%, with an adsorption capacity at equilibrium of 6.42 mg/g. The adsorption of MV6B followed a pseudo-second-order kinetic model and the Freundlich model isotherm. A thermodynamic study showed that the adsorption process was spontaneous and exothermic. PAL/Fe3O4 was highly reusable after three cycles without the need for desorption. Hence, this study has demonstrated that the PAL/ Fe3O4 adsorbent is practical, economical, and efficient for wastewater treatment.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Aljeddani G, Alghanmi R, Hamouda R. Study on the isotherms, kinetics, and thermodynamics of adsorption of crystal violet dye using Ag-NPs-loaded cellulose derived from peanut-husk agro-waste. Polymers 2023;15(22):Article No. 4394.
Al-Hazmi G, Akhdhar A, Shahat A, Elwakeel K. Adsorption of Gentian violet dye onto mesoporous aluminosilica monoliths: Nanoarchitectonics and application to industrial wastewater. International Journal of Environmental Analytical Chemistry 2022. DOI: https://doi.org/10.1080/03067319.2022.2104641.
Ali N, Jabbar N, Alardhi S, Majdi H, Albayati T. Adsorption of methyl violet dye onto a prepared bio-adsorbent from date seeds: Isotherm, kinetics, and thermodynamics studies. Heliyon 2022;8(8):Article No. 10276.
AL-Shehri H, Almudaifer E, Alorabi A, Alanazi H, Alkorbi A, Alharthi F. Effective adsorption of crystal violet from aqueous solutions with effective adsorbent: Equilibrium, mechanism studies and modeling analysis. Environmental Pollutants and Bioavailability 2021;33(1):214-26.
Ayawei N, Angaye S, Wankasi D, Dikio E. Synthesis, characterization and application of Mg/Al layered double hydroxide for the degradation of congo red in aqueous solution. Open Journal of Physical Chemistry 2015;5(3):56-70.
Ayawei N, Ebelegi A, Wankasi D. Modelling and interpretation of adsorption isotherms. Journal of Chemistry 2017;2017:Article No. 3039817.
Beigi P, Ganjali F, Hassanzadeh-Afruzi F, Salehi M, Maleki A. Enhancement of adsorption efficiency of crystal violet and chlorpyrifos onto pectin hydrogel@Fe3O4-bentonite as a versatile nanoadsorbent. Scientific Reports 2023;13:Article No. 10764.
Benhalima T, Ferfera-Harrar H. Eco-friendly porous carboxymethyl cellulose/dextran sulfate composite beads as reusable and efficient adsorbents of cationic dye methylene blue. International Journal of Biological Macromolecules 2019;132:126-41.
Benhalima T, Ferfera-Harrar H, Lerari D. Optimization of carboxymethyl cellulose hydrogels beads generated by an anionic surfactant micelle templating for cationic dye uptake: Swelling, sorption and reusability studies. International Journal of Biological Macromolecules 2017;105(1):1025-42.
Benhalima T, Ferfera-Harrar H, Saha N, Saha P. Fe3O4 imbuing carboxymethyl cellulose/dextran sulfate nanocomposite hydrogel beads: An effective adsorbent for methylene blue dye pollutant. Journal of Macromolecular Science, Part A 2023a;60(6):442-61.
Benhalima T, Chicha W, Ferfera-Harrar H. Sponge-like biodegradable polypyrrole-modified biopolymers for selective adsorption of basic red 46 and crystal violet dyes from single and binary component systems. International Journal of Biological Macromolecules 2023b;253(8):Article No. 127532.
Benhalima T, Mokhtari M, Ferfera-Harrar H. Synergistic adsorption/photodegradation effect for effective removal of crystal violet dye and acetamiprid pesticide using Fe3+ cross-linked ternary carboxymethyl cellulose/polyaniline/TiO2 photocomposites. Journal of Water Process Engineering 2024;57:Article No. 104670.
Bensalah J. Removal of the textile dyes by a resin adsorbent polymeric: Insight into optimization, kinetics and isotherms adsorption phenomenally. Inorganic Chemistry Communications 2024;161:Article No. 111975.
Bensalah B, Idrissi A, Faydy M, Doumane G, Staoui A, Hsissou R, et al. Investigation of the cationic resin as a potential adsorbent to remove MR and CV dyes: Kinetic, equilibrium isotherms studies and DFT calculations. Journal of Molecular Structure 2023;1278:Article No. 134849.
Canbaz G. Fe3O4@Granite: A novel magnetic adsorbent for dye adsorption. Processes 2023;11(9):Article No. 2681.
Casas N, Parella T, Vicent T, Caminal G, Sarrà M. Metabolites from the biodegradation of triphenylmethane dyes by Trametes versicolor or laccase. Chemosphere 2009; 75(10):1344-9.
Chaudhari A. Nitrobenzene oxidation for isolation of value added products from industrial waste lignin. Journal of Chemical, Biological and Physical Sciences 2016;6(63):501-13.
Chen J, Chen H. Removal of anionic dyes from an aqueous solution by a magnetic cationic adsorbent modified with DMDAAC. New Journal of Chemistry 2018;42:7262-71.
Chiban M, Soudani A, Sinan F, Persin M. Single, binary and multi-component adsorption of some anions and heavy metals on environmentally friendly Carpobrotus edulis plant. Colloids Surf B Biointerfaces 2011;82(2):267-76.
Fang L, Wu H, Shi Y, Tao Y, Yong Q. Preparation of lignin-based magnetic adsorbent from kraft lignin for adsorbing the congo red. Frontiers in Bioengineering and Biotechnology 2021;9:Article No. 691528.
Foroutan R, Mohammadi R, Farjadfard S, Esmaeili H, Ramavandi B, Sorial G. Eggshell nano-particle potential for methyl violet and mercury ion removal: Surface study and field application. Advanced Powder Technology 2019;30(10):2188-99.
Foroutan R, Peighambardoust S, Peighambardoust S, Pateiro M, Lorenzo J. Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/Fe3O4 magnetic nanocomposite from Aqueous Solutions: A kinetic, equilibrium and thermodynamic study. Molecules 2021; 26(8):Article No. 2241.
Kamdod A, Kumar M. Adsorption of methylene blue, methyl orange, and crystal violet on microporous coconut shell activated carbon and its composite with chitosan: Isotherms and kinetics. Journal of Polymers and the Environment 2022;30:5274-89.
Khadam S, Javed T, Jilani M. Adsorptive exclusion of crystal violet dye from wastewater using eggshells: Kinetic and thermodynamic study. Desalination and Water Treatment 2022;268:99-112.
Liu G, Liao L, Dai Z, Qi Q, Wu J, Ma L, et al. Organic adsorbents modified with citric acid and Fe3O4 enhance the removal of Cd and Pb in contaminated solutions. Chemical Engineering Journal 2020;39:Article No. 125108.
Liu L, Luo X, Ding L, Luo S. Application of nanotechnology in the removal of heavy metal from water. In: Liu L, Luo X, Ding L, Luo S, Deng F, editors. Nanomaterials for Nanomaterials for the Removal of Pollutants and Resource Reutilization. Elsevier; 2019. p. 83-147.
Meng X, Scheidemantle B, Li M, Wang Y, Zhao X, Toro-González M, et al. Synthesis, characterization, and utilization of a lignin-based adsorbent for effective removal of azo dye from aqueous solution. ACS Omega 2020;5(6):2865-77.
Panamgama A, Peramune P. Coconut coir pith lignin: A physicochemical and thermal characterization. International Journal of Biological Macromolecules 2018;113:1149-57.
Ringot D, Lerzy B, Chaplain K, Bonhoure J, Auclair E, Larondelle Y. In vitro biosorption of ochratoxin A on the yeast industry by-products: Comparison of isotherm models. Bioresource Technology 2007;98(9):1812-21.
Saini R. Textile organic dyes: Polluting effects and elimination methods from textile waste water. International Journal of Chemical Engineering Research 2017;9(1):121-36.
Sharma J, Sharma S, Soni V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Regional Studies in Marine Science 2021;45:Article No. 101802.
Sultana S, Islam K, Hasan M, Khan H, Khan M, Deb A, et al. Adsorption of crystal violet dye by coconut husk powder: Isotherm, kinetics and thermodynamics perspectives. Environmental Nanotechnology, Monitoring and Management 2022;17:Article No. 100651.
Taleb F, Ammar M, Mosbah M, Salem R, Moussaoui Y. Chemical modification of lignin derived from spent coffee grounds for methylene blue adsorption. Scientific Reports 2020;10:Article No. 11048.
Touihri M, Guesmi F, Hannachi C, Hamrouni B, Sellaoui L, Badawi M, et al. Single and simultaneous adsorption of Cr (VI) and Cu (II) on a novel Fe3O4/pine cones gel beads nanocomposite: Experiments, characterization and isotherms modeling. Chemical Engineering Journal 2021;416:Article No. 129101.
Wang T, Jiang M, Yu X, Niu N, Chen L. Application of lignin adsorbent in wastewater Treatment: A review. Separation and Purification Technology 2022;302:Article No. 122116.
You X, Zhou R, Zhu Y, Bu D, Cheng D. Adsorption of dyes methyl violet and malachite green from aqueous solution on multi-step modified rice husk powder in single and binary systems: Characterization, adsorption behavior and physical interpretations. Journal of Hazardous Materials 2022; 430:Article No. 128445.
Younesi-Kordkheili H, Pizzi A. A Comparison among lignin modification methods on the properties of lignin-phenol-formaldehyde resin as wood adhesive. Polymers 2021; 13(20):Article No. 3502.