Study of Crystal Structure, Lattice Strain, and Elemental Content of Natural Iron Sand Nanoparticles Synthesized by the Coprecipitation Method 10.32526/ennrj/23/20240141
Main Article Content
Abstract
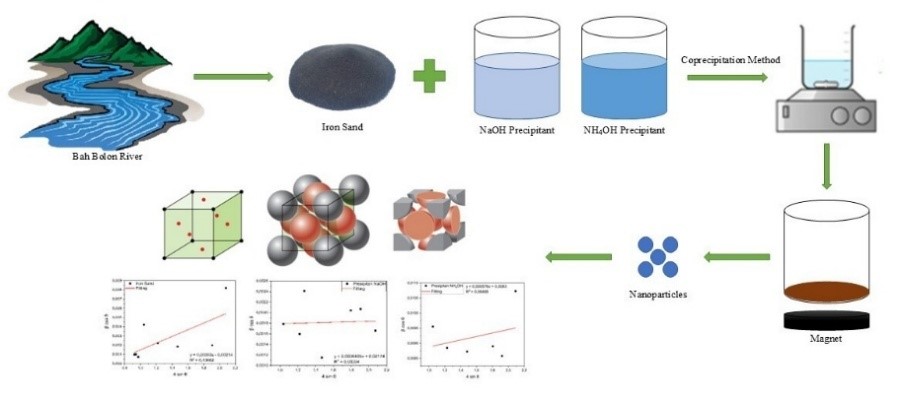
This study was conducted to investigate the synthesis of magnetite nanoparticles from iron sand collected from the Bah Bolon River in Indonesia, using the coprecipitation method with NaOH and NH4OH as precipitants. The results showed that based on SEM-EDX (scanning electron microscopy coupled with energy-dispersive x-ray spectroscopy) analysis, the Fe content of the raw iron sand, initially at 34.76%, increased to 45.50% following synthesis with NH4OH, indicating enhanced purity in the final product. SEM observations found average particle sizes of approximately 53 nm for nanoparticles synthesized with NaOH and 20 nm for those synthesized with NH4OH. X-ray diffraction (XRD) analysis confirmed that the synthesized nanoparticles retain the magnetite (Fe3O4) phase with a face-centered cubic (FCC) spinel structure. Crystallite size calculations using the Scherrer equation yielded average crystallite sizes of 80.194 nm for NaOH-synthesized samples and 15.124 nm for NH4OH-synthesized samples, demonstrating that NH4OH favors the formation of smaller crystallites. Lattice strain analysis through the Williamson-Hall method showed positive tensile strain values for all samples, indicating structural tension within the crystal lattice. The NH4OH-synthesized nanoparticles had slightly higher lattice strain, suggesting that synthesis conditions impact both crystallite size and lattice tension. In conclusion, this study demonstrated that NH4OH was more effective than NaOH in producing high-purity, small-crystallite magnetite nanoparticles from natural iron sand, with potential implications for enhanced material properties.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Abdel-Mohsen LH, Lafta SH, Hashim MS. Comparing the role of NaOH and NH4OH on structural and magnetic properties of spinel ba ferrite synthesized by autocombustion method. Journal of Physics: Conference Series 2022;2322:Article No. 012081.
Al-Salih M, Samsudin S, Arshad SS. Synthesis and characterizations iron oxide carbon nanotubes nanocomposite by laser ablation for anti-microbial applications. Journal of Genetic Engineering and Biotechnology 2021;19(1):Article No. 76.
Alkallas FH, Alghamdi SM, Rashed EA, Trabelsi ABG, Nafee SS, Elsharkawy WB, et al. Nanocomposite Fe3O4-MWCNTs based on femtosecond pulsed laser ablation for catalytic degradation. Diamond and Related Materials 2023;140(PA):Article No. 110445.
Amiruddin E, Prayitno A. The synthesis of magnetic nanoparticles from naturaliron sand of Kata Beach Pariaman West Sumatera using ball milling method as environmental material. MATEC Web of Conferences 2019;276:Article No. 06014.
Ba-Abbad MM, Benamour A, Ewis D, Mohammad AW, Mahmoudi E. Synthesis of Fe3O4 nanoparticles with different shapes through a co-precipitation method and their application. Journal of the Minerals, Metals and Materials Society 2022;74(9):3531-9.
Calderón Bedoya PA, Botta PM, Bercoff PG, Fanovich MA. Magnetic iron oxides nanoparticles obtained by mechanochemical reactions from different solid precursors. Journal of Alloys and Compounds 2021;860:Article No. 157892
Dadashi S, Poursalehi R, Delavari H. Structural and optical properties of pure iron and iron oxide nanoparticles prepared via pulsed Nd:YAG laser ablation in liquid. Procedia Materials Science 2015;11:722-6.
Díez AG, Rincón-Iglesias M, Lanceros-Méndez S, Reguera J, Lizundia E. Multicomponent magnetic nanoparticle engineering: The role of structure-property relationship in advanced applications. Materials Today Chemistry 2022;26: Article No. 101220.
Elaoud A, Mechi A, Tlili H, Ferhi M, Hassen H Ben. Green synthesis and characterization of magnetite nanoparticles using eucalyptus globulus leaves for water treatment and agronomic valorization. Environmental Monitoring and Assessment 2024;196(9):Article No. 786.
Fatimah S, Juharni J, Wahyuni ASH. Green synthesis of Fe3O4 nanoparticles based on natural sand as methylene blue degradation by electrocoagulation method. Positron 2023;13(1):86-94.
Girardet T, Venturini P, Martinez H, Dupin JC, Cleymand F, Fleutot S. Spinel magnetic iron oxide nanoparticles: Properties, synthesis and washing methods. Applied Sciences 2022;12(16):Article No. 8127.
Gutierrez FV, Lima IS, De Falco A, Ereias BM, Baffa O, Diego de Abreu Lima C, et al. The effect of temperature on the synthesis of magnetite nanoparticles by the coprecipitation method. Heliyon 2024;10(4):e25781.
Hamid M, Rianna M, Rangkuti WR, Sembiring T, Sebayang P. Study and characterization RGO/Fe3O4 in microstructure and magnetic properties. South African Journal of Chemical Engineering 2022;42:280-2.
Hamid M, Susilawati, Amaturrahim SA, Dalimunthe IB, Daulay A. Synthesis of magnetic activated carbon-supported cobalt(II) chloride derived from pecan shell (Aleurites Moluccana) with co-precipitation method as the electrode in supercapacitors. Materials Science for Energy Technologies 2023;6:429-36.
Hu P, Chang T, Chen WJ, Deng J, Li SL, Zuo YG, et al. Temperature effects on magnetic properties of Fe3O4 nanoparticles synthesized by the sol-gel explosion-assisted method. Journal of Alloys and Compounds 2019;773:605-11.
Humaidi S, Hamid M, Wijoyo H. Study and characterization of BaFe12O19-PVDF materials prepared by co-precipitation for supercapacitor electrodes applications. Authorea 2023; DOI: 10.22541/au.169887066.61389194/v1.
Jain R, Kumar S, Meena SK. Precipitating agent (NaOH and NH4OH) dependent magnetic properties of cobalt ferrite nanoparticles. AIP Advances 2022;12:Article No. 095109.
Mardana IBP, Lutfiyah YN, Yasa P, Widiantara GKA. Synthesis and characterization of magnetite Fe3O4 nanoparticles from natural iron sand in Gelar River. Indonesian Physical Review 2023;6(1):114-23.
Melinia LA, Puspita E, Naibaho M, Ramlan R, Ginting M. Analysis of natural iron sand from Musi River, South Sumatra. Journal of Scientific Research 2022;24(3):Article No. 122.
Mustapha S, Ndamitso MM, Abdulkareem AS, Tijani JO, Shuaib DT, Mohammed AK, et al. Comparative study of crystallite size using williamson-hall and debye-scherrer plots for ZnO nanoparticles. Advances in Natural Sciences: Nanoscience and Nanotechnology 2019;10(4):Article No. 045013.
Nguyen MD, Tran H-V, Xu S, Lee TR. Fe3O4 nanoparticles : structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Applied Sciences 2021;11:Article No. 11301.
Novita N, Naibaho M, Puspita E, Ramlan R, Ginting M, Humaidi S. Analysis of mineral content and magnetic properties of iron sand of Bah Bolon Simalungun River, North Sumetera. Asian Journal of Engineering, Social and Health 2023;2(12):1633-9.
Novita, Ramlan, Naibaho M, Ginting M, Humaidi S, Duma TN. Fe2O3 review: Nanostructure, synthesis methods, and applications. International Journal of Social Service and Research 2024;04(02):539-59.
Parvathy Namboothiri PM, Vasundhara M. Synthesis and characterization of nano-hematite. Materials Today: Proceedings 2023;92:1459-63.
Patel K, Patel A, Jethwa VP, Patel H, Solanki GK. X-Ray diffraction analysis of orthorhombic SnSe nanoparticles by Williamson-Hall, Halder-Wagner and Size-Strain plot methods. Chemical Physics Impact 2024;8:Article No. 100547.
Polla MB, Nicolini JL, Venturini J, da Cas Viegas A, Zen Vasconcellos MA, Montedo ORK, et al. Low-temperature sol-gel synthesis of magnetite superparamagnetic nanoparticles: influence of heat treatment and citrate-nitrate equivalence ratio. Ceramics International 2023;49(5):7322-32.
Prasetyowati R, Widiawati D, Swastika PE, Ariswan A, Warsono W. Synthesis and characterization of magnetite (Fe3O4) nanoparticles based on iron sands at Glagah Beach Kulon Progo with coprecipitation methods at various NH4OH concentrations. Journal of Basic Science 2021;10(2):57-61.
Rianna M, Hamid M, Handayani F, Sebayang AMS, Rangkuti WR, Situmorang M, et al. Study and characterization of Fe3O4 synthesized from natural iron sand in Sumatera Utara. Journal of Aceh Physics Society 2022;11(2):45-8.
Sinaga JEE, Budianto G, Pritama VL, Suhendra. Particle Size and Lattice Strain Effect on the Optical Constants of Fe3O4 Nanoparticles. Indonesian Physical Review 2023;6(1):114-23.
Taufiq A, Nikmah A, Hidayat A, Sunaryono S, Mufti N, Hidayat N, et al. Synthesis of magnetite/silica nanocomposites from natural sand to create a drug delivery vehicle. Heliyon 2020;6(4):e03784.
Tlili H, Elaoud A, Asses N, Horchani-naifer K, Ferhi M, Goya GF, et al. Reduction of oxidizable pollutants in waste water from the Wadi El Bey River Basin using magnetic nanoparticles as removal agents. Magnetoche 2023;9(6):Article No. 157.
Tukan DN, Rosmainar L, Kustomo K, Rasidah R. A review: optimum conditions for magnetite synthesis (Fe3O4). Journal of Scientific Periodicals of Science and Applied Chemistry 2023;17(2):Article No. 15.
Wang W, Li F, Li S, Hu Y, Xu M, Zhang Y, et al. M2 macrophage-targeted iron oxide nanoparticles for magnetic resonance image-guided magnetic hyperthermia therapy. Journal of Materials Science and Technology 2021;81:77-87.
Yuwanda AN, Rahmayuni R, Visgun DA, Rahmi A, Rifai H, Dwiridal L. Characterization of magnetic minerals of iron sand Pasia Nan Tigo Padang Beach using X-Ray diffraction (XRD). Indonesian Journal of Applied Physics 2022;12(1):35-47.
Zhang W, Zhang Z, Lou S, Chang Z, Wen B, Zhang T. Hyaluronic acid-stabilized Fe3O4 nanoparticles for promoting In vivo magnetic resonance imaging of tumors. Frontiers in Pharmacology 2022;13:Article No. 918819.