Performance and Heavy Metal Leaching Behavior of Bituminous Fly Ash-Based Geopolymer in Aggressive Environments 10.32526/ennrj/23/20240170
Main Article Content
Abstract
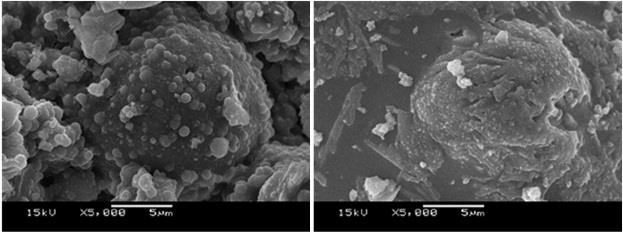
This article investigates the performance of a geopolymer synthesized from bituminous fly ash (BFA) activated with sodium hydroxide. The BFA-based geopolymer (BFAG) exhibited high mechanical strength and a densified microstructure. The optimal SiO2/Al2O3 and Na2O/SiO2 molar ratios were found to be 3:1 and 0.2, respectively, yielding a 28-day compressive strength of 9.65 MPa. The inclusion of 30wt.% of a PS material containing heavy metals led to a substantial reduction in strength by 56% and 73% compared to samples with the SiO2/Al2O3 molar ratio of 2:1 and 3:1, respectively, at 28 days. The ability of the BFAG matrix to contain 30wt.% PS was evaluated using a waste extraction test (WET). The leaching behavior of heavy metals from the BFAG matrix was assessed with three aggressive leachants: sodium citrate, sodium acetate, and synthetic acid rain. Results showed that, under exposure to these leachants, the leached concentrations of Cr, Fe, and Zn from samples with the SiO2/Al2O3 ratio of 3:1 were lower than those from samples with the 2:1 ratio, with concentrations ranging from 0.32-1.73, 3.07-6.67, and 152-284 mg/L, respectively. Despite exposure to harsh conditions, the BFAG matrix effectively immobilized over 99% for Cr and Fe and Zn, except when exposed to sodium citrate which only retained 98.5% of Zn. BFAG can be used to treat heavy metal and heavy metal-contaminated sludge. This matrix reduces environmental exposure, thereby decreasing heavy metal leaching into the environment before safe disposal in landfills. BFAG can also be used as a cement substitute in the solidified process, which lowers treatment costs and reduces cement consumption. It can decrease carbon dioxide emissions from cement production.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Aly Z, Vance ER, Perera DS, Hanna JV, Griffith CS, Davis J, et al. Aqueous leachability of metakaolin-based geopolymers with molar ratios of Si/Al=1.5-4. Journal of Nuclear Materials 2008;378(2):172-9.
Amran M, Debbarma S, Ozbakkaloglu T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Construction and Building Materials 2021;270:Article No. 121857.
Asavapisit S, Macphee DE. Immobilization of metal-containing waste in alkali-activated lime-RHA cementitious matrices. Cement and Concrete Research 2007;37(5):776-80.
Askarian M, Tao Z, Samali B, Adam G, Shuaibu R. Mix composition and characterisation of one-part geopolymers with different activators. Construction and Building Materials 2019;225:526-37.
Castillo H, Collado H, Droguett T, Sánchez S, Vesely M, Garrido P, et al. Factors affecting the compressive strength of geopolymers: A review. Minerals 2021;11(12):Article No. 1317.
Castillo H, Collado H, Droguett T, Vesely M, Garrido P, Palma S. State of the art of geopolymers: A review. e-Polymers 2022;22(1):108-24.
Cheng H, Lin KL, Cui R, Hwang CL, Chang YM, Cheng TW. The effects of SiO2/Na2O molar ratio on the characteristics of alkali-activated waste catalyst-metakaolin based geopolymers. Construction and Building Materials 2015;95:710-20.
Cong P, Cheng Y. Advances in geopolymer materials: A comprehensive review. Journal of Traffic and Transportation Engineering (English Edition) 2021;8(3):283-314.
Duxson P, Provis JL, Lukey GC, Mallicoat SW, Kriven WM, Deventer JSJ. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2005;269(1-3):47-58.
Elmesalami N, Celik K. A critical review of engineered geopolymer composite: A low-carbon ultra-high-performance concrete. Construction and Building Materials 2022; 346:Article No. 128491.
Fan C, Wang B, Ai H, Qi Y, Liu Z. A comparative study on solidification/stabilization characteristics of coal fly ash-based geopolymer and Portland cement on heavy metals in MSWI fly ash. Cleaner Production 2021;319:Article No. 128790.
Lombi E, Stevens DP, McLaughlin MJ. Effect of water treatment residuals on soil phosphorus, copper and aluminium availability and toxicity. Environmental Pollution 2010; 158(6):2110-16.
Luhar I, Luhar S. A comprehensive review on fly ash-based geopolymer. Journal of Composites Science 2022;6(8):Article No. 219.
Provis JL, Deventer JSJ. Geopolymers: Structure, Processing, Properties and Industrial Applications. Cambridge, United Kingdom: Woodhead Publishing; 2009.
Shi P, Zhang Y, Sun Q, Ta X. Eluviation and leaching of elements from broken fly-ash-based porous geopolymer. Materials 2021;14(22):Article No. 6884.
Silva P, Crenstil KS, Sirivivatnanon V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cement and Concrete Research 2007;37(4):512-8.
Su Y, Luo B, Luo Z, Xu F, Huang H, Long Z, et al. Mechanical characteristics and solidification mechanism of slag/fly ash-based geopolymer and cement solidified organic clay: A comparative study. Journal of Building Engineering 2023;71:Article No. 106459.
Sun Z, Tang Q, Xakalashe BS, Fan X, Gan M, Chen X, et al. Mechanical and environmental characteristics of red mud geopolymers. Construction and Building Materials 2022;321:Article No. 125564.
Teixeira SR, Santos GTA, Souza AE, Alessio P, Souza SA, Souza NR. The effect of incorporation of a Brazilian water treatment plant sludge on the properties of ceramic materials. Applied Clay Science 2011;53(4):561-5.
Tian Q, Bai Y, Pan Y, Chen C, Yao S, Sasaki K, et al. Application of geopolymer in stabilization/solidification of hazardous pollutants: A Review. Molecules 2022;27(14):Article No. 4570.
Valencia-Saavedra WG, Mejía de Gutiérrez R, Puertas F. Performance of FA-based geopolymer concretes exposed to acetic and sulfuric acids. Construction and Building Materials 2020;275:Article No. 119503.
Van Jaarsveld JGS, Van Deventer FSJ. The effect of metal contaminants on the formation and properties of waste-based geopolymers. Cement and Concrete Research 1999;29(8): 1189-200.
Waijarean N, MacKenzie KJD, Asavapisit S, Piyapanuwat R, Jameson GNL. Synthesis and properties of geopolymers based on water treatment residue and their immobilization of some heavy metals. Journal of Materials Science 2017;52:7345-59.
Xia M, Muhammad F, Li S, Lin H, Huang X, Jiao B, et al. Solidification of electroplating sludge with alkali-activated fly ash to prepare a non-burnt brick and its risk assessment. The Royal Society of Chemistry 2020;10:4640-9.
Yang M, Zheng Y, Li X, Yang X, Rao F, Zhong L. Durability of alkali-activated materials with different C-S-H and N-A-S-H gels in acid and alkaline environment. Materials Research and Technology 2022;16:619-30.
Zhang J, Provis JL, Feng D, Van Deventer JSJ. Geopolymers for immobilization of Cr+6, Cd+2, and Pb+2. Journal of Hazardous Materials 2008;157(2-3):587-98.
Zheng L, Wang W, Shi Y. The effects of alkaline dosage and Si/Al ration on the immobilization of heavy metals in municipal solid waste incineration fly ash-based geopolymer. Chemosphere 2010;79(6):665-71.