Does predation risk affect body size and shape ontogeny in the silver barb (Barbonymus gonionotus)?
Main Article Content
Abstract
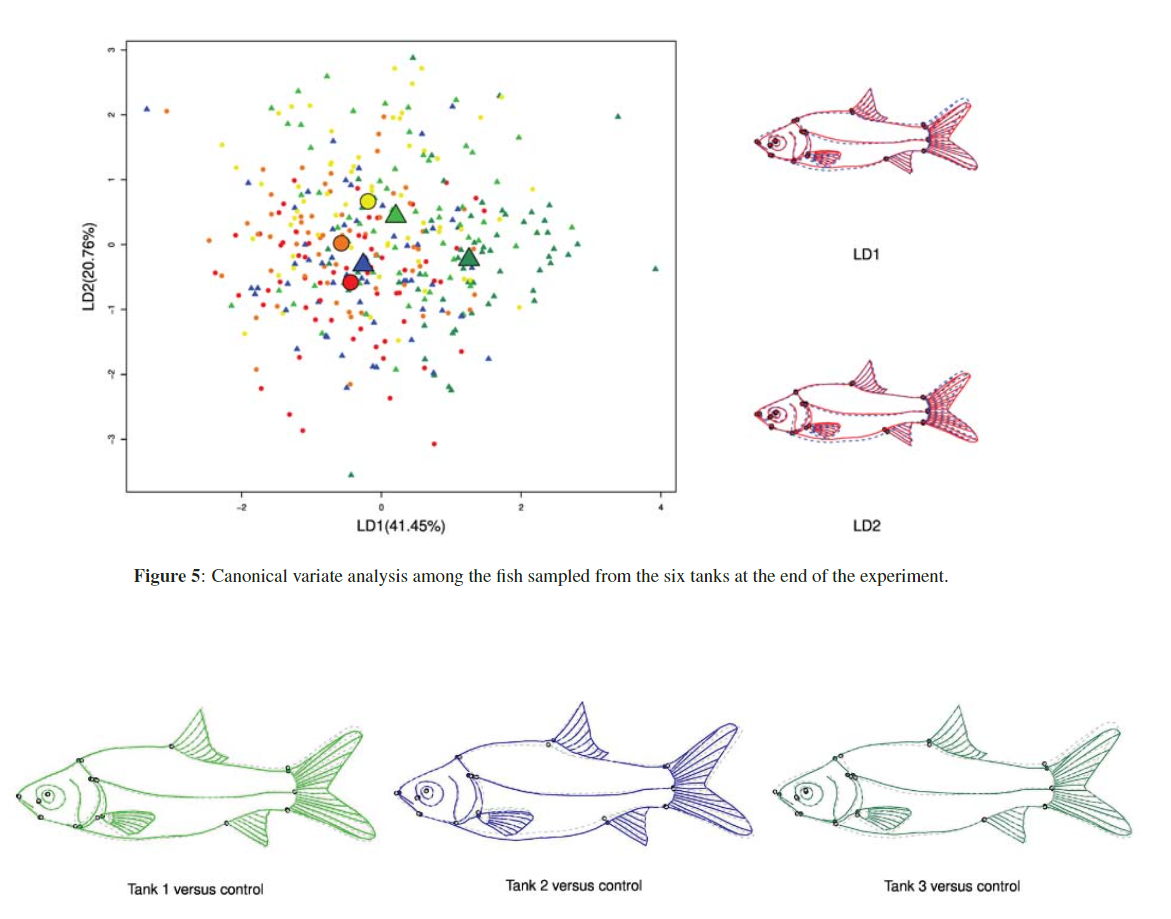
We studied here the effect of predation risk on size and shape during the development of the Cyprinid fish (Barbonymus gonionotus). In the experiment, juvenile silver barbs (Barbonymus gonionotus) were developing either together or not together with the predator snakehead fish (Channa striata) during 25 days. Predation was limited by isolating the predator from the silver barb with a net. In replicated trays, 60 fish were randomly selected and compared before and after the experiment in presence and absence of the predator. The experiment was replicated three times. Fourteen landmarks were recorded on the fish body and a generalized Procrustes superimposition was performed. Analyses of variance and linear discriminant analyses were used to detect effects of the predator presence on body shape and growth pattern. Results show that if presents, effect of the predator on size and shape evolution in silver barbs is very subtle. A small increase of size and a decrease in relative caudal peduncle height could be reported in all cases suggesting either that the predator could exert directional selection or that developmental plasticity induced by the predator was present. In the case of shape, this developmental plasticity appears to be maladaptive in the experiment because the predator may have selected for these shape attributes in the tank showing the highest predation rate. Finally we found that shape variation decreased with development suggesting that phenotypic canalisation was acquired during the ontogeny of the fish but that size differences among individuals were accumulating with ontogeny.
Article Details
References
C. K. Ghalambor, J. K. McKay, S. P. Carroll, D. N. Reznick, Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments, Functional Ecology 21(2017) 394–407.
A. A. Agrawal, Phenotypic plasticity in the interactions andevolution of species, Science 294(2001) 321–326.
D. P. Chivers, R. J. F. Smith, Chemical alarm signallingin aquatic predator prey systems: A review and prospectus, ́Ecoscience 5(1998) 338–352.
O. B. Stabell, M. S. Lwin, Predator-induced phenotypicchanges in crucian carp are caused by chemical signalsfrom conspecifics, Environmental Biology of Fishes 49(1997)145–149.
J. Van Buskirk, B. R. Schmidt, Predator induced phenotypic plasticity in larval newts: Tradeoffs, selection, and variation innature, Ecology 81(2000) 3009–3028.
B. G. Miner, S. E. Sultan, S. G. Morgan, D. K. Padilla, R.A. Relyea, Ecological consequences of phenotypic plasticity,Trends in Ecology and Evolution 20(2005) 685–692.
J. A. Endler , Multipletrait coevolution and environmental gradients in guppies, Trends in Ecology and Evolution 10(1995)22–29.
C. A. Handelsman, E. D. Broder, C. M. Dalton, E. W. Ruell, C.A. Myrick, D. N. Reznick, C. K. Ghalambor, Predator induced phenotypic plasticity in metabolism and rate of growth: Rapidadaptation to a novel environment, Integrative and ComparativeBiology 53(2013) 975–988.
C. A. Handelsman, E. W. Ruell, J. Torres Dowdall, C. K. Ghalambor, Phenotypic plasticity changes correlations of traits following experimental introductions of Trinidadian guppies(Poe-cilia reticulata), Integrative and Comparative Biology 54(2014)794–804.
S. K. J. McConnell , Mapping aquatic faunal exchanges acrossthe Sunda shelf, South East Asia, using distributional and genetic data sets from the Cyprinid fishBarbodes gonionotus(Bleeker, 1850), Journal of Natural History 38(2004) 651–670.
D. V. Thinh, N. S. Van, T. H. T. Nguyen,Barbonymus gonionotus[Internet], IUCN Red List Threat.Species 2012. 2012 [cited 2019 Mar 15], Available from:http://dx.doi.org/10.2305/IUCN.UK.2012-1.RLTS.T166914A1151554.en.
W. J. Rainboth, Fishes of the Cambodian Mekong. FAO species identification field guide for fishery purposes, Rome: Food and Agriculture Organization of the United Nations(1996) 265.
FAO, Aquaculture production 1985–1994. FAO Fisheries Circular, (1996)
A. J. Rothuis, L. T. Nam, C. J. J. Richter, F. Ollevier, Polyculture of silver barb, Puntius gonionotus (Bleeker), Nile tilapia,Oreochromis niloticus (L.), and common carp, Cyprinus carpio L., in Vietnamese ricefields: fish production parameters. Aquaculture Research 2(1998) 661–668.
Department of Fisheries. Department of Fisheries [Internet], [cited 2019 Mar 15], Available from: https://www4.fisheries.go.th/index.php/dofen.
A. K. A. Rahman, Freshwater fishes of Bangladesh. Zoological Society of Bangladesh. Department of Zoology, University of Dhaka, (1989)
W. Wongboonma, Survival rate of juvenile silver barbs (Barbonymus gonionotus)after rearing with predators and without predators, Senior Project, Department of Biology, Faculty ofScience, Burapha University, (2015) [In Thai]
I. L. Dryden, K. V. Mardia, Statistical shape analysis, London:John Wiley (1998)
R. Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, (2019). Available from: https://www.R-project.org.
J. Claude, Morphometrics with R. New York, NY: Springer Verlag, (2008).
S. M. Yezerinac, S. C. Lougheed, P. Handford, Measurement error and morphometric studies: Statistical power and observere experience, Systematic Biology 41(1992) 471–482.
J. Claude, Log shape ratios, Procrustes super imposition, elliptic Fourier analysis: Three worked examples in R. Hystrix, the Italian Journal of Mammalogy 24(2013) 94–102.
C. Goodall, Procrustes methods in the statistical analysis of shape, Journal of the Royal Statistical Society Series B(Methodological) 5(1991) 285–339.
D. Bates, M. M ̈achler, B. Bolker, S. Walker, Fitting linear mixed effects models using lme 4, Journal of Statistical Software (2015) 67.
J. Oksanen, F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. OHara, G. L. Simpson, P. Solymos, M. H. M. Stevens, H. Wagner, Package “vegan”: community ecology package. 2019; http://vegan.r-forge.r-project.org/. Available from: https://cran.r-project.org/web/packages/vegan/vegan.pdf.
B. B. Chapman, L. J. Morrell, T. G. Benton, J. Krause, Earlyinter actions with adults mediate the development of predator defenses in guppies, Behavioral Ecology 19(2008) 87–93.
J. Torres Dowdall, C. A. Handelsman, D. N. Reznick, C. K. Ghalambor, Local adaptation and the evolution of phenotypic plasticity in Trinidadian guppies (Poecilia reticulata), Evolution 66(2012) 3432–3443.
K. L. Feilich, G. V. Lauder, Passive mechanical models of fishcaudal fins: Effects of shape and stiffness on self propulsion, Bioinspiration and Biomimetics 10(2015) 36002.
P. W. Webb, Body Form, Locomotion and Foraging in Aquatic Vertebrates, American Zoologist 24(1984) 107–120.
K. J. Parsons, S. Sk ́ulason, M. Ferguson, Morphological variation over ontogeny and environments in resource polymorphic arctic charr (Salvelinus alpinus), Evolution & Development 12(2010) 246–257.