Modified mangosteen pericarp powder as a biosorbent for removal of methyl violet 2B from aqueous solutions
Main Article Content
Abstract
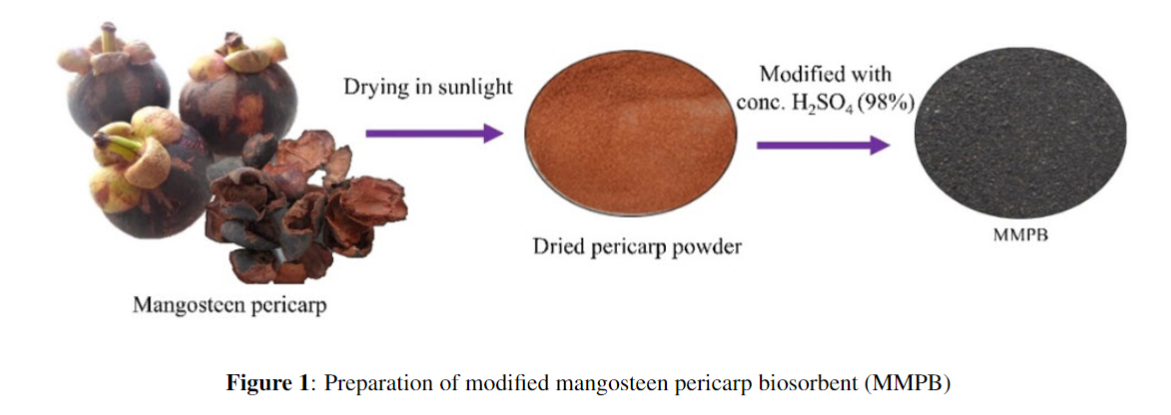
This paper proposes the removal of methyl violet 2B from aqueous medium using modified mangosteen pericarp (MMPB) as biosorbent. The characteristic of surface biosorbent was examined by SEM and FTIR. It found that surface morphology was porous structure and demonstrated various functional groups which advantage for cationic dye adsorption. Effect of pH, amount of biosorbent and contact time on the dye removal efficiency were tested by batch experiments. The results indicated that the percentage of dye elimination increased with increasing of pH, amount of biosorbent and contact time. The experimental data showed a removal efficiency of the dye was higher than 95% from 10 mg/L of initial MV2B concentration with the pH range of 6 to 10 using amounts of biosorbent as 0.05 g in 25 mL dye solution and contact time of 90 min. The adsorption isotherm was well fitted to Langmuir model (R2 = 0.982) with a maximum adsorption capacity (qm) of 49.75 mg/g, suggesting the monolayer onto a homogenous surface. This study implied that modified mangosteen pericarp can be employed in the act of an inexpensive biosorbent for methyl violet 2B removal from aqueous solutions.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Research and Markets.com," Global $28.88 Billion Synthetic Dyes Markets, Analysis, Opportunities and Strategies, 2015-2020, 2025F, 2030FW”, Available from: ttps://www.businesswire.com/ news/ home/ 20220512005748/en/Global-28.88-Billion-Synthetic-Dyes-Markets-Analysis-Opportunities-and-Strategies-2015-2020-2025F-2030F (accessed on May 12, 2022).
Slama H.B., A. C. Bouket, Z. Pourhassan, F. N. Alenezi, A. Silini, H. Cherif-Silini, T. O. Luptakova, L.P. Golińska and L.Belbahri , Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods, Applied sciencea 11(2021) 6255. https://doi.org/10.3390/app11146 255
Lellis B., Fávaro-Polonio C. Z.,Pamphile J. Al., Polonio J. C., Effects of textile dyes on health and the environment and bioremediation potential of living organisms, Biotechnology Research and Innovation 3(2019) 275-290. https://doi.org/10.1016/j.biori.2019.09.001
Musyoka S. M., Mittal H., Mishra S.B., and Ngila J. C., Effect of functionalization on the adsorption capacity of cellulose for the removal of methyl violet, International Journal of Biological Macromolecules 65(2014)389–397. http://dx.doi.org/10.1016/j.ijbiomac.2014.01.051
Rahchamani J., H. Zavvar Mousavi, and M. Behzad, Adsorption of methyl violet from aqueous solution by polyacrylamide as an adsorbent: Isotherm and kinetic studies, Desalination 267(2011) 256–260. doi:10.1016/j.desal.2010.09.036
Mitrogiannis D., G. Markou, A. Çelekli, and H. Bozkurt, Biosorption of methylene blue onto Arthrospira platensis biomass: Kinetic, equilibrium and thermodynamic studies, Journal of Environ mental Chemical Engineering, 3(2015) 670–680. http://dx.doi.org/10.1016/j.jece.2015.02.008
Foroutan R., R. Mohammadi, S. Farjadfard, H. Esmaeili, B. Ramavandi,and G. A. Sorial, Eggshell nano-particle potential for methyl violet and mercury ion removal: Surface study and field applica- tion. Advanced Powder Technology 30 (2019) 2188–2199. https://doi.org/10.1016/j.apt.2019.06.034
Al-Tohamy R., S.S.Ali, F.Li, K.M.Okasha, Y.A.G. Mahmoud, T. Elsamahy, H. Jiao, Y. Fu, and J. Sun, A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety, Ecotoxico- logy and Environmental Safety 231(2022)113160. https://doi.org/10.1016/j.ecoenv.2021.113160
Yagub M.T.,T. K.Sen, S.Afroze, and H.M. Ang, Dye and its removal from aqueous solution by adsorption: A review, Advances in Colloid and Interface Science,209 (2014)172–184. http://dx.doi. org/10.1016/j.cis.2014. 04.002
Astuti W., A. Chafidz, E.T.Wahyuni, A.Prasetya, I. M. Bendiyasa, and A. E. Abasaeed, Methyl violet dye removal using coal fly ash (CFA) as a dual sites adsorbent, Journal of Environmental Chemical Engineering 7(2019)103262. https://doi.org/10.1016/j.jece.2019.103262
Augustine E. Ofomaja, Emmanuel E. Ukpebor, Stephen A. Uzoekwe, Biosorption of Methyl violet onto palm kernel fiber: Diffusion studies and multistage process design to minimize biosorbent mass and contact time, Biomass and Bioenergy 35(2011)4112-4123. doi:10.1016/j.biombioe.2011.05.024
Elgarahy A.M., K.Z. Elwakeel , S.H. Mohammad, and G.A. Elshoubaky, A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process, Cleaner Engineering and Technology 4(2021)100209. https://doi.org/10.1016/j.clet.2021.100209
Kulkarni M. R., T. Revanth, A. Acharya, and P. Bhat, Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study, Resource-Efficient Technologies3(2017)71–77. http://dx.doi.org/10.1016/j.reffit.2017.01.009
Fomina M., and G. M. Gad, Biosorption: current perspectives on concept, definition and application, Bioresource Technology160(2014).3-14. http://dx.doi.org/10.1016/j.biortech.2013.12.102
Loulidi I., F. Boukhlifi, M. Ouchabi, A. Amar, M. Jabri, A. Kali, S Chraibi, C. Hadey, and F. Aziz, Adsorption of Crystal Violet onto an Agricultural Waste Residue: Kinetics, Isotherm, Thermodyna- mics, and Mechanism of Adsorption, The Scientific World Journal (2020) Article ID 5873521. https://doi.org/ 10.1155/ 2020/ 5873521
Arfi R. B., S. Karoui, K. Mougin and A. Ghorbal, Adsorptive removal of cationic and anionic dyes from aqueous solution by utilizing almond shell as biosorbent, Euro-Mediterranean Journal for Environmental Integration (EMJE) 2(20)(2017)1-13. doi:10.1007/s41207-017-0032-y
Al-Ghouti M. A. and S.S. Dib, Utilization of nano-olive stones in environmental remediation of methylene blue from water, Journal of Environmental Health Science and Engineering18 (2020) 63–77. https://doi.org/ 10.1007/s40201-019-00438-y
Kooh M. R. R. , M. K. Dahri and L. B. L. Lim, Removal of the methyl violet 2B dye from aqueous solution using sustainable adsorbent Artocarpus odoratissimus stem axis, Applied Water Science 7(2017)3573–3581.doi 10.1007/s13201-016-0496-y
Andjani D., I. Sriyanti, A. Fauzi, D. Edikresnha, M. M.l Munir, H. Rachmawati, and Khairurrijal, Rotary Forcespun Polyvinylpyrrolidone (PVP) Fibers as a Mangosteen Pericarp Extracts Carrier, In proceeding of Engineering Physics International Conference, Procedia Engineer ing170(2017) 14-18. doi:10.1016/j.proeng.2017.03.003
Jaisupa N., P. Moongkarndi, P. Lomarat, J. Samer, V. Tunrungtavee,W. Muangpaisan , and S. Mangmool, Mangosteen peel extract exhibits cellular antioxidant activity by induction of catalase and heme oxygenase-1 mRNA expression, Journal of Food Biochemistry 42(2018)e12511. https://doi.org/10.1111/jfbc.12511
Chen Y., B. Huang, M. Huang,and B. Cai,On the preparation and characterization of activated carbon from mangosteen shell, Journal of the Taiwan Institute of Chemical Engineers 42(2011) 837-842. doi:10.1016/j. jtice.2011.01.007
Kurniawan Y. S., M. R. G. Fahmi, and L. Yuliati, Isolation and Optical Properties of Natural Pigments from Purple Mangosteen Peels, In proceeding of The 2nd International Conference on Chemistry and Material Science (IC2MS) IOP Conf. Series: Materials Science and Engineering 833(2020)012018. doi:10.1088/1757-899X/833/ 1/ 012018
Silva C. E. F., B. M. V. Gama, A. H. S. Gonçalves, J. A. Medeiros, and A. K. S. Abud, Basic-dye adsorption in albedo residue: Effect of pH, contact time, temperature, dye concentration, biomass dosage, rotation and ionic strength, Journal of King Saud University–Engineering Sciences 32 (2020) 351–359. https://doi.org/ 10. 1016/j.jksues.2019.04.006
Zhang Z., L. Xu, Y. Liu , R. Feng, T. Zou, Y. Zhang, Y. Kang, and P. Zhou , Efficient removal of methylene blue using the mesoporous activated carbon obtained from mangosteen peel wastes: Kinetic, equilibrium, and thermodynamic studies, Microporous and Mesoporous Materials 315 (2021) 110904. https://doi.org/10.1016/ j. micromeso.2021.110904
Zein R., R. Suhaili, F. Earnestly, Indrawati, and E. Munaf, Removal of Pb(II), Cd(II) and Co(II) from aqueous solution using Garcinia mangostana L. fruit shell, Journal of Hazardous Materials181(2010) 52–56.doi: 10.1016/j.jhazmat.2010.04.076
Kooh M. R.R., M. K. Dahri, and L.B. L. Lim , Removal of methyl violet 2B dye from aqueous solution using Nepenthes rafflesiana pitcher and leaves, Applied Water Science7(2017) 3859–3868. doi10.1007/s13201-017-0537-1
Ooi J., L.Y. Lee, B. Y. Z. Hiew, S. Thangalazhy-Gopakumar, S. S. Lim, and S. Gan, Assessment offish scales waste as a low cost and eco-friendly adsorbent for removal of an azo dye: equilibrium, kinetic and thermodynamic studies, Bioresource Technology245(2017) 656-664. http://dx.doi.org/ 10.1016/j.biortech.2017.08.153