Chitosan/Poly(vinyl alcohol)/Collagen Hydrogel Composites Containing Jackfruit Axis Extract for Wound Dressing Application
Main Article Content
Abstract
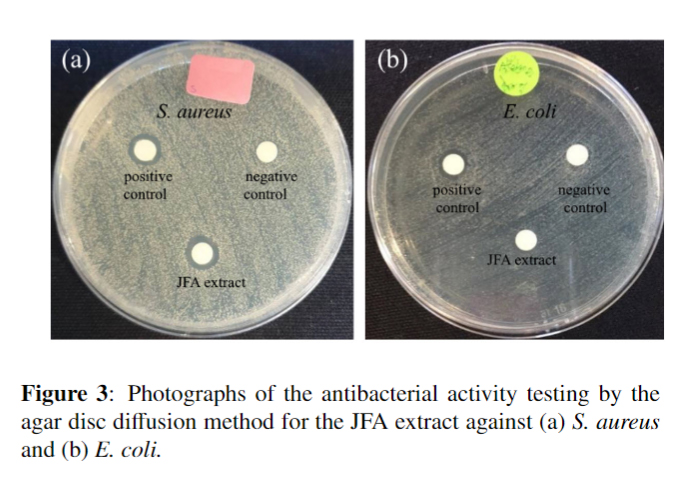
Jackfruit (Artocarpus heterophyllus Lam.) axis (JFA) was extracted using sonication in ethanol. The yield of extraction was about 4.93%. The films of chitosan (CS)/poly(vinyl alcohol) (PVA)/collagen (Coll) hydrogel composites containing JFA extract were prepared from the mixed solutions of 1% w/v CS, 1% w/v PVA, and Coll at various ratios including 5/4/1, 5/3/2, 4/5/1, and 4/4/2 by weight of solution. The JFA extract was added into the mixed solution at 0.25% w/w. A solvent casting was performed followed by crosslinking via glutaraldehyde vapor treatment. The obtained films were named as JFA-CS/PVA/Coll 5/4/1, 5/3/2, 4/5/1, and 4/4/2. The actual JFA extract content was 19 ± 3.6% based on the weight of dry film. Antioxidant activity of JFA extract was evaluated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. The half-maximal inhibitory concentration (IC50) of JFA extract was 0.250 mg/mL. The JFA extract only exhibited antibacterial activity against Staphylococcus aureus (S. aureus), but not for Escherichia coli (E. coli), as determined by an agar disc diffusion method. The release of JFA extract from the hydrogel composite films was studied by total immersion method in distilled water at 37°C during 0-8 h. The JFA-CS/PVA/Coll 4/4/2 showed higher amounts of JFA extract released than those from the ratios of 4/5/1, 5/3/2, and 5/4/1, respectively. The degree of water retention and weight loss of the films appeared in a similar trend to those of the release study. The higher content of CS and lower content of Coll led to the lower amounts of water retention, weight loss, and JFA extract release. Lastly, all types of JFA-CS/PVA/Coll films exhibited antioxidant activity of about 46-51% and antibacterial activity against S. aureus. However, the JFA-CS/PVA/Coll 5/4/1 showed the least antioxidant and antibacterial activities. Based on the overall results, the JFA-CS/PVA/Coll films revealed the potential for use in wound dressing applications.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
L. Fan, H. Yang, J. Yang, M. Peng, J. Hu, Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings, Carbohydrate polymers, 146 (2016) 427-434.
K. Kalantari, E. Mostafavi, B. Saleh, P. Soltantabar, T.J. Webster, Chitosan/PVA hydrogels incorporated with green synthesized cerium oxide nanoparticles for wound healing applications, European Polymer Journal, 134 (2020) 109853.
E.A. Kamoun, E.-R.S. Kenawy, X. Chen, A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings, Journal of advanced research, 8 (2017) 217-233.
S. Kumaraswamy, S.H. Mallaiah, Swelling and mechanical properties of radiation crosslinked Au/PVA hydrogel nanocomposites, Radiation Effects and Defects in Solids, 171 (2016) 869-878.
A. Gupta, R. Kumar, N. Upadhyay, P. Surekha, P. Roy, Synthesis, characterization and efficacy of chemically crosslinked PVA hydrogels for dermal wound healing in experimental animals, Journal of Applied Polymer Science, 111 (2009) 1400-1408.
I. Patacho, A.S. Oliveira, P. Nolasco, R. Colaço, A.P. Serro, Chemically crosslinked PVA hydrogels for cartilage substitution, Annals of Medicine, 53 (2021) S19-S19.
S. Vineeth, R.V. Gadhave, P.T. Gadekar, Glyoxal Cross-linked polyvinyl alcohol-microcrystalline cellulose blend as a wood adhesive with enhanced mechanical, thermal and performance properties, Mater Int, 2 (2020) 0277-0285.
H.S. Mansur, C.M. Sadahira, A.N. Souza, A.A. Mansur, FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde, Materials Science and Engineering: C, 28 (2008) 539-548.
D. Altiok, E. Altiok, F. Tihminlioglu, Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications, Journal of Materials Science: Materials in Medicine, 21 (2010) 2227-2236.
M. Hadidi, S. Pouramin, F. Adinepour, S. Haghani, S.M. Jafari, Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities, Carbohydrate polymers, 236 (2020) 116075.
M.J. Moreno-Vásquez, E.L. Valenzuela-Buitimea, M. Plascencia-Jatomea, J.C. Encinas-Encinas, F. Rodríguez-Félix, S. Sánchez-Valdes, E.C. Rosas-Burgos, V.M. Ocaño-Higuera, A.Z. Graciano-Verdugo, Functionalization of chitosan by a free radical reaction: Characterization, antioxidant and antibacterial potential, Carbohydrate Polymers, 155 (2017) 117-127.
C.N. Nandana, M. Christeena, D. Bharathi, Synthesis and characterization of chitosan/silver nanocomposite using rutin for antibacterial, antioxidant and photocatalytic applications, Journal of Cluster Science, 33 (2022) 269-279.
R. Huang, W. Li, X. Lv, Z. Lei, Y. Bian, H. Deng, H. Wang, J. Li, X. Li, Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing, Biomaterials, 53 (2015) 58-75.
H. Xie, X. Chen, X. Shen, Y. He, W. Chen, Q. Luo, W. Ge, W. Yuan, X. Tang, D. Hou, Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing, International journal of biological macromolecules, 107 (2018) 93-104.
K.C. Loureiro, T.C. Barbosa, M. Nery, M.V. Chaud, C.F. da Silva, L.N. Andrade, C.B. Corrêa, A. Jaguer, F.F. Padilha, J.C. Cardoso, Antibacterial activity of chitosan/collagen membranes containing red propolis extract, Die Pharmazie-An International Journal of Pharmaceutical Sciences, 75 (2020) 75-81.
M. Barbălată-Mândru, D. Serbezeanu, M. Butnaru, C.M. Rîmbu, A.A. Enache, M. Aflori, Poly (vinyl alcohol)/Plant Extracts Films: Preparation, Surface Characterization and Antibacterial Studies against Gram Positive and Gram Negative Bacteria, Materials, 15 (2022) 2493.
A.U. Khan, I.J. Ema, M. Faruk, S.A. Tarapder, A.U. Khan, S. Noreen, M. Adnan, A review on importance of Artocarpus heterophyllus L.(Jackfruit), Journal of Multidisciplinary Applied Natural Science, (2021).
R. Ranasinghe, S. Maduwanthi, R. Marapana, Nutritional and health benefits of jackfruit (Artocarpus heterophyllus Lam.): a review, International journal of food science, 2019 (2019).
N. Dhwani, G. Raju, S.E. Mathew, G. Baranwal, S.B. Shivaram, N. Katiyar, N. Pramanik, S. Jhunjhunwala, H. Shilpashree, D.A. Nagegowda, Antibacterial efficacy of Jackfruit rag extract against clinically important pathogens and validation of its antimicrobial activity in Shigella dysenteriae infected Drosophila melanogaster infection model, bioRxiv, (2020).
Z. Li, Y. Lan, J. Miao, X. Chen, B. Chen, G. Liu, X. Wu, X. Zhu, Y. Cao, Phytochemicals, antioxidant capacity and cytoprotective effects of jackfruit (Artocarpus heterophyllus Lam.) axis extracts on HepG2 cells, Food Bioscience, 41 (2021) 100933.
H. Cortes, I.H. Caballero-Florán, N. Mendoza-Muñoz, L. Escutia-Guadarrama, G. Figueroa-González, O.D. Reyes-Hernández, M. González-Del Carmen, M. Varela-Cardoso, M. González-Torres, B. Florán, Xanthan gum in drug release, Cellular and Molecular Biology, 66 (2020) 199-207.
W. Sutananta, D.Q. Craig, J.M. Newton, An evaluation of the mechanisms of drug release from glyceride bases, Journal of pharmacy and pharmacology, 47 (1995) 182-187.
A. Çay, M. Miraftab, E.P.A. Kumbasar, Characterization and swelling performance of physically stabilized electrospun poly (vinyl alcohol)/chitosan nanofibres, European Polymer Journal, 61 (2014) 253-262.
Y. Zhou, D. Yang, J. Nie, Effect of PVA content on morphology, swelling and mechanical property of crosslinked chitosan/PVA nanofibre, Plastics, rubber and composites, 36 (2007) 254-258.
W. Lan, M. Xu, X. Zhang, L. Zhao, D. Huang, X. Wei, W. Chen, Biomimetic polyvinyl alcohol/type II collagen hydrogels for cartilage tissue engineering, Journal of Biomaterials Science, Polymer Edition, 31 (2020) 1179-1198.