Fast and Efficient Removal of Hexavalent Chromium from Water by Iron Oxide Particles
Main Article Content
Abstract
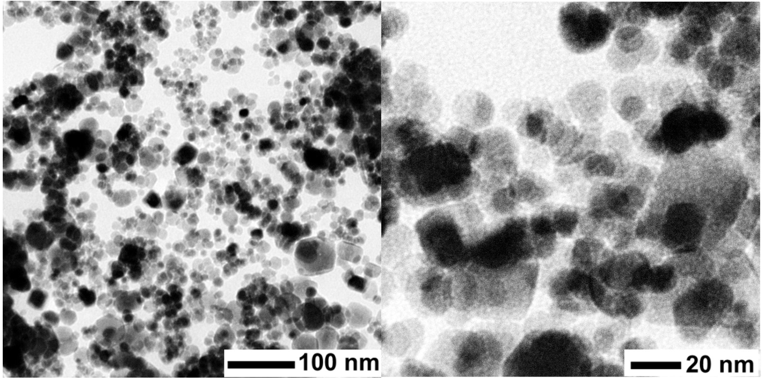
Iron oxide particles (IOPs) were synthesized by chemical co-precipitation technique and further used as an adsorbent in removing hexavalent chromium (Cr(VI)) from aqueous solutions during batch adsorption. The IOP adsorbent had specific surface area of 65 m2/g, total pore volume of 0.25 cm3/g and mostly contained a mesoporous structure. The analysis of scanning and transmission electron microscopy indicated that the adsorbent contained a substantial amount of iron oxide of about 66%, which was well distributed throughout the adsorbent. The IOP adsorbent showed a rapid and efficient Cr(VI) removal that followed Langmuir adsorption isotherm model with maximum adsorption capacity of 2.39 mg-Cr(VI)/g-IOP, demonstrating a monolayer formation on the adsorptive sites of IOP. The kinetic adsorption of Cr(VI) on the IOP followed the pseudo-second-order model, suggesting chemisorption. Thus, the IOP adsorbent provides a potentially effective technology in eliminating of Cr(VI) from water since it can remove appreciable amounts of Cr(VI) with a relatively short contact time of 30 min.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.