Adsorption of Reactive Dyes from Wastewater Using Cationic Surfactant-modified Coffee Husk Biochar DOI: 10.32526/ennrj.18.1.2020.03
Main Article Content
Abstract
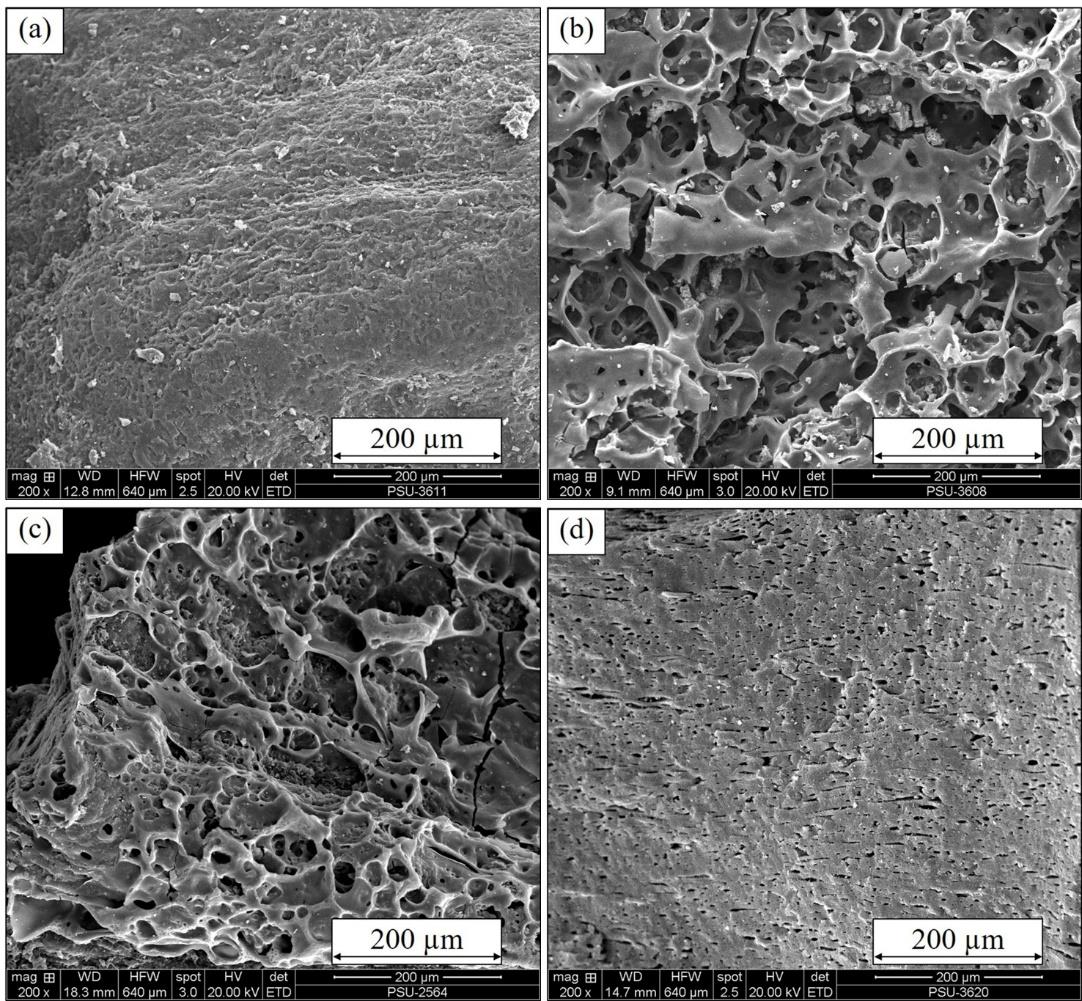
A solid agricultural waste, coffee husk, was applied as an adsorbent for reactive dye-polluted wastewater treatment. Coffee husk biochar was pyrolyzed at 450 °C and then chemically activated using 50% ZnCl2 solution. The surface of activated coffee husk biochar was modified using a cationic surfactant, Cetyltrimethylammonium bromide (CTAB), to create CTAB-modified coffee husk biochar (MCH), to improve reactive adsorption of anionic dyes from synthetic wastewater. The selected reactive dyes were reactive yellow 145
(RDY145), reactive red 195 (RDR195), and reactive blue 222 (RDB222). The adsorption kinetics fit well using a pseudo-second order model for all three dyes. The adsorption isotherms matched well with the Langmuir model . The removal efficiency of RDY145 (83.7%) was the highest, followed by RDR195 (71.1%) and RDB222 (59.6%). The amount of RDY145 adsorbed by MCH was about 9-fold that adsorbed by conventional activated carbon. Additionally, the solution pH had no effect on reactive dye removal efficiency using MCH.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
2. Amin NK. Removal of reactive dye from aqueous solutions by adsorption onto activated carbons prepared from sugarcane bagasse pith. Desalination 2008;223(1-3):152-61.
3. Brito MJP, Veloso CM, Santos LS, Bonomo RCF, Fontan R da CI. Adsorption of the textile dye Dianix® royal blue CC onto carbons obtained from yellow mombin fruit stones and activated with KOH and H3PO4: kinetics, adsorption equilibrium and thermodynamic studies. Powder Technology 2018;339:334-43.
4. Department of Industrial Works, Ministry of Industry. Wastewater Management Guidance Manual from Textile Factories. Bangkok, Thailand: Department of Industrial Works, Ministry of Industry; 2013.
5. de Franco MAE, de Carvalho CB, Bonetto MM, Soares RD, Féris LA. Removal of amoxicillin from water by adsorption onto activated carbon in batch process and fixed bed column: kinetics, isotherms, experimental design and breakthrough curves modelling. Journal of Cleaner Production 2017;161:947-56.
6. GEO-Informatics Research Center for Natural Resource and Environment, Southern Regional Center of Geo-Informatics and Space Technology. Area of Tham Sing sub-district in Mueang Chumphon District, Chumphon Province, Thailand. Songkhla, Thailand: Prince of Songkla University; 2019.
7. Ghazi Mokri HS, Modirshahla N, Behnajady MA, Vahid B. Adsorption of C.I. acid red 97 dye from aqueous solution onto walnut shell: kinetics, thermodynamics parameters, isotherms. International Journal of Environmental Science and Technology 2015;12(4): 1401-8.
8. Guidechem. Reactive Red 195 [Internet]. 2017 [cited 2019 Feb 26]. Available from: https://www.guidechem. com/reference/dic-276579.html#Properties.
9. Ibrahim S, Fatimah I, Ang HM, Wang S. Adsorption of anionic dyes in aqueous solution using chemically modified barley straw. Water Science and Technology 2010;62(5):1177-82.
10. Ip AWM, Barford JP, McKay G. A comparative study on the kinetics and mechanisms of removal of reactive black 5 by adsorption onto activated carbons and bone char. Chemical Engineering Journal 2010;157(2-3):434-42.
11. Ip AWM, Barford JP, McKay G. Reactive black dye adsorption/desorption onto different adsorbents: effect of salt, surface chemistry, pore size and surface area. Journal of Colloid and Interface Science 2009; 337(1):32-8.
12. Krivova MG, Grinshpan DD, Hedin N. Adsorption of CnTABr surfactants on activated carbons. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2013;436:62-70.
13. Kula I, Uğurlu M, Karaoğlu H, Çelik A. Adsorption of Cd(II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation. Bioresource Technology 2008;99(3):492-501.
14. Lin S-Y, Chen W-f, Cheng M-T, Li Q. Investigation of factors that affect cationic surfactant loading on activated carbon and perchlorate adsorption. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2013;434:236-42.
15. Maleangpoothong J, Photha P, Siriwongyotha C, Paksa W, Rommanee W, Taweesuk D, Srichoo C. Thailand experiences from the grassroots: value chain finance best practices, initiatives, strategies and trends in agriculture. Bangkok, Thailand: Asia-Pacific Rural and Agricultural Credit Association; 2013.
16. Markandeya, Shukl SP, Dhiman N. Characterization and adsorption of disperse dyes from wastewater onto cenospheres activated carbon composites. Environmental Earth Sciences 2017;76(20):1-12.
17. Meng L, Xu X, Bai B, Ma M, Li S, Hu N, Wang H, Suo Y. Surface carboxyl-activated polyester (PET) fibers decorated with glucose carbon microspheres and their enhanced selective adsorption for dyes. Journal of Physics and Chemistry of Solids 2018;123:378-88.
18. Mi X, Li G, Zhu W, Liu L. Enhanced adsorption of orange II using cationic surfactant modified biochar pyrolyzed from cornstalk. Journal of Chemistry 2016;2016:1-7.
19. Moazzam A, Jamil N, Nadeem F, Qadir A, Ahsan N, Zameer M. Reactive dye removal by a novel biochar/MgO nanocomposite. Journal of the Chemical Society of Pakistan 2017;39(1):26-34.
20. Mook WT, Aroua MK, Szlachta M. Palm shell-based activated carbon for removing reactive black 5 dye: equilibrium and kinetics studies. BioResources 2016;11(1):1432-47.
21. Mozammel HM, Masahiro O, Bhattacharya SC. Activated charcoal from coconut shell using ZnCl2 activation. Biomass and Bioenergy 2002;22(5):397-400.
22. Nabil GM, El-mallah NM, Mahmoud ME. Enhanced decolorization of reactive black 5 dye by active carbon sorbent-immobilized-cationic surfactant (AC-CS). Journal of Industrial and Engineering Chemistry 2014;20(3):994-1002.
23. National Center for Biotechnology Information (NCBI). PubChem Compound Database; CID=5974 [Internet]. 2004 [cited 2018 Jul 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/5974.
24. National Center for Biotechnology Information (NCBI). PubChem Compound Database; CID=157317 [Internet]. 2005 [cited 2018 Jul 20]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/157317.
25. National Center for Biotechnology Information (NCBI). PubChem Compound Database; CID=102407104 [Internet]. 2015 [cited 2018 Jul 20]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/102407104.
26. Oei BC, Ibrahim S, Wang S, Ang HM. Surfactant modified barley straw for removal of acid and reactive dyes from aqueous solution. Bioresource Technology 2009;100(18):4292-5.
27. Ogugbue CJ, Sawidis T. Bioremediation and detoxification of synthetic wastewater containing triarylmethane dyes by aeromonas hydrophila isolated from industrial effluent. Biotechnology Research International 2011;2011:1-11.
28. Puasa SW, Ismail KN, Khairi NAIA. Direct surfactant-impregnated activated carbon for adsorption of reactive blue 4. International Journal of Engineering and Technology 2018;7(4):5-8.
29. Rehman HA, Razzaq R. Benefits of biochar on the agriculture and environment: a review. Journal of Environmental Analytical Chemistry 2017;4(3):1-3.
30. Su Y, Zhao B, Xiao W, Han R. Adsorption behavior of light green anionic dye using cationic surfactant-modified wheat straw in batch and column mode. Environmental Science and Pollution Research 2013;20(8):5558-68.
31. Sun D, Zhang Z, Wang M, Wu Y. Adsorption of reactive dyes on activated carbon developed from Enteromorpha prolifera. American Journal of Analytical Chemistry 2013;4(7A):17-26.
32. Thitame PV, Shukla SR. Adsorptive removal of reactive dyes from aqueous solution using activated carbon synthesized from waste biomass materials. International Journal of Environmental Science and Technology 2016;13(2):561-70.
33. Vijayaraghavan K, Won SW, Yun Y-S. Treatment of complex Remazol dye effluent using sawdust- and coal-based activated carbons. Journal of Hazardous Materials 2009;167(1-3):790-6.
34. Wang X, Jiang C, Hou B, Wang Y, Hao C, Wu J. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 2018;206:587-96.
35. Zhang R, Zhang J, Zhang X, Dou C, Han R. Adsorption of Congo red from aqueous solutions using cationic surfactant modified wheat straw in batch mode: kinetic and equilibrium study. Journal of the Taiwan Institute of Chemical Engineers 2014;45(5):2578-83.
36. Zhao B, Xiao W, Shang Y, Zhu H, Han R. Adsorption of light green anionic dye using cationic surfactant-modified peanut husk in batch mode. Arabian Journal of Chemistry 2017;10(Supplement 2):S3595-602.