Study of Ribulose 1, 5-Bisphosphate Carboxylase from Sulfobacillus acidophilus Strain NY-1 Isolated from Lignite Mines DOI: 10.32526/ennrj.18.4.2020.34
Main Article Content
Abstract
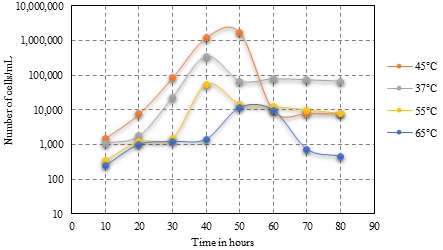
One of the key compounds engaged in carbon dioxide fixation cycle (Calvin-Benson-Bassham cycle) is Ribulose 1, 5- BisphosphateCarboxylase/Oxygenase (RuBisCo). These acts as a carbon sink thus leading to decrease of carbon level. This one of a kind property of RuBisCo thus can help in diminishing an Earth-wide global warming, a noteworthy risk in the present world. In the present study, presence of RuBisCo in Sulfobacillus isolated from Neyveli lignite mines was explored. The growth factors such as pH, Temperature, aeration and light source were optimized for the growth of Sulfobacillus acidophilus strain NY-1. The ideal pH and temperature for Sulfobacillus was seen at pH 1.7 and at 45oC respectively. The cell count was greatest under light condition of 1.60 x 106 at the 60th and 2.96 X 106 in the presence of CO2 with aeration. The presence of RuBisCo under optimized conditions was affirmed by a simple ion exchange chromatography technique.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Sage, R.F., Way, D. A., Kubien, D. S., Rubisco. Rubisco activase and global climate change. Journal of Experimental Botany 2008; 59 (7): 1581-95.
Meenakshi, S., Srisudha, S. In silico Characterization and Homology Modeling of Cyanobacterial RuBisCo (LS) with Computational Tools and Bioinformatic Servers. Helix 2012; 4: 185-191.
Ke-Quing, X., Peng, B., Qiong-Li, B., Yan, J., Fu-Yi, H., Jian-Qiang, S., Yong-Guan, Z., Quantitative analyses of ribulose-1, 5-bisphosphate Carboxylase/Oxygenase (RuBisCo) large-subunit genes (cbbL) in typical paddy soils. Federation of European Microbiological Societies Microbiology Ecology 2014; 87: 89-101.
Ken, K., 1 Norihiro, M., Toshiaki, F., Haruyuki, A., Tadayuki, I., Kunio, M. Crystal Structure of a Novel-Type Archaeal RuBisCo with Pentagonal Symmetry. Structure 2001; 9(6): 473-481.
Caldwell, P. E., MacLean, M. R., Norris, P. R. Ribulose Bisphosphate Carboxylase activity and a Calvin cycle gene cluster in Sulfobacillus species. Microbiology 2007; 153: 2231–2240.
Fischer, R.A., Edmeades, G. O. Breeding and cereal yield progress. Crop Science 2010; 50: 85–98.
Tobias J Erb and Jan Zarzycki. A short history of Rubisco: the rise and fall of Nature’s predominant CO2 fixing enzyme. Current Opinion in Biotechnology 2018; 49: 100-107.
Delwiche, C. F., Palmer, J. D. Rampant horizontal transfer and duplication of RuBisCo genes in eubacteria and plastids. Molecular Biology and Evolution 1996; 13: 873-882.
Watson, G. M. F., Tabita, F. R. Microbial Ribulose 1, 5-bisphosphate Carboxylase/Oxygenase: a molecule for phylogenetic and enzymological investigation. Federation of European Microbiological Societies Microbiology Letters 1997; 146:13-22.
Newman, J., Gutteridge, S. Structure of an effector-induced inactivated state of ribulose-1, 5-bisphosphate Carboxylase/Oxygenase: the binary complex between enzyme and xylulose 1, 5-bisphosphate. Structure 1994; 2: 495-502.
Nakagawa, H., Sugimoto, M., Kai, Y., Harada, S., Miki, K., Kasai, N. Preliminary crystallographic study of a ribulose-1, 5-bisphosphate Carboxylase Oxygenase from Chromatium vinosum. Journal of Molecular Biology 1986; 191: 577-578.
Schneider, G., Lindqvist, Y., Lundqvist, T. Crystallographic renement and structure of Ribulose- 1,5-bisphosphate Carboxylase from Rhodospirillum rubrum at 1.7 AE resolution. Journal of Molecular Biology 1990; 211: 989-1008.
Shibata, N., Yamamoto, H., Inoue, T., Uemura, K., Yokota, A., Kai, Y. Crystallization and preliminary crystallographic studies of ribulose-1,5-bisphosphate Carboxylase/Oxygenase from a red alga, Galdieria partita, with a high specificity factor. Journal of Biochemistry 1996; 120: 1064-1066.
Andersson, I., Knight, S., Schneider, G., Lindqvist, Y., Lundqvist, T., BraÈndeÂn, C.-I., Lorimer, G. H. Crystal structure of the active site of Ribulose- Bisphosphate Carboxylase. Nature 1989; 337: 229- 234.
Chapman, M. S., Suh, S. W., Curmi, P. M., Cascio, D., Smith, W. W. & Eisenberg, D. S. Tertiary structure of plant RuBisCo: domains and their contacts. Science 1988; 241: 71-74.
Portis, Archie & Parry, Martin. Discoveries in RuBisCo (Ribulose 1, 5-bisphosphate Carboxylase/Oxygenase): A historical perspective. Photosynthesis research 2007; 94: 121-43. 10.
Chakrabarty, S., Bhattacharya, S., Bhattacharya, K. A nonradioactive assay method for determination of enzymatic activity of -ribulose-1, 5-bisphosphate Carboxylase/Oxygenase (RuBisCo). Journal of Biochemical and Biophysical Methods 2002; 52; 179-187.
Watling, H. R., Perrot, F. A., Shiers, D. W., Comparison of selected characteristics of Sulfobacillus species and review of their occurrence in acidic and bioleaching environments. Hydrometallurgy 2008; 93: 57–65.
Simon, P., Robinson, V., Streusand, J., Mark, C., Archie, R., Portis. Purification and Assay of RuBisCo Activase from Leaves. Plant Physiology 1988; 88: 1008-1014.
Kaneda, T. Iso and ante isofatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiological Reviews 1991; 5; 288–302.
Tsaplina, I. A., Bogdanova, T. I., Sayakin, D. D., Karavaiko, G. I. Effects of organic substances on the growth of Sulfobacillus thermosulfidooxidans and pyrite oxidation. Mikrobiologiya 1991; 60: 686–692.
Norris, P. A., Clark, D. A., Owen, J. P., Waterhouse, S., Characteristics of Sulfobacillus acidophilus sp. nov. and other moderately thermophilic mineral-sulphide- oxidizing bacteria. Microbiology 1996; 142: 775–783.
Golovacheva R. S., Karavaiko, G.I. Sulfobacillus, a new genus of thermophilic sporulating bacteria. Mikrobiologiia 1978; 47(5): 815-822.
Wedel, N., Soll, J. Evolutionary conserved light regulation of Calvin cycle activity by NADPH-mediated reversible phosphoribulokinase/CP12/glyceraldehyde-3-phosphate dehydrogenase complex dissociation. Proceedings of the National Academy of Sciences of the United States of America 1998; 95: 9699–9704.