Potential of Palm Kernel Alkanolamide Surfactant for Enhancing Oil Recovery from Sandstone Reservoir Rocks DOI: 10.32526/ennrj.18.4.2020.32
Main Article Content
Abstract
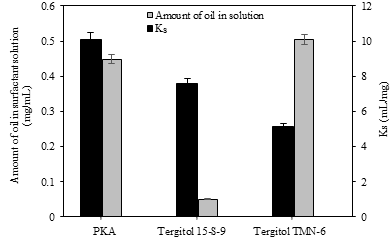
The interest in using benign surfactants has been steadily increasing in the context of enhanced oil recovery (EOR). Palm kernel alkanolamide surfactant (PKA), a nonionic surfactant synthesized from palm kernel oil, was preliminarily assessed for EOR from sandstone reservoir rocks. The performance factors determined were silica adsorption for surfactant loss and crude oil solubilization for oil solubilizing efficiency. The performance of PKA was compared to two commercial ionic surfactants, SDS (anionic surfactant) and CTAB (cationic surfactant). The results show that PKA was less absorbed on silica than CTAB or SDS. The adsorption kinetics were well fit with a pseudo-second order model for all three surfactants. The adsorption equilibrium data for CTAB and PKA were fitted with Langmuir isotherm, while for SDS Freundlich isotherm fit well, indicating multilayer SDS adsorption on silica surfaces. The adsorption of PKA was not significantly affected by added salt or increased temperature. In addition, the solubilization equilibrium constant (Ks) had the rank order PKA > CTAB > SDS, and proportionally increased with added salt. PKA performance was also compared to two commercial nonionic surfactants, Tergitol 15-S-9 and Tergitol TMN-6, and the results indicate that PKA was the least adsorbed, and had the highest Ks among the tested nonionic surfactants.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Ahmadi MA, Shadizadeh SR. Experimental investigation of a natural surfactant adsorption on shale-sandstone reservoir rocks: Static and dynamic conditions. Fuel 2015;159:15–26.
Ahmadi MA, Zendehboudi S, Shafiei A, James L. Nonionic surfactant for enhanced oil recovery from carbonates: Adsorption kinetics and equilibrium. Ind Eng Chem Res 2012;51:9894–905.
Arabloo M, Ghazanfari MH, Rashtchian D. Spotlight on kinetic and equilibrium adsorption of a new surfactant onto sandstone minerals: A comparative study. J Taiwan Inst Chem Eng 2015;50:12–23.
Atia AA, Radwan NRE. Adsorption of different surfactants on kaolinite. Adsorpt Sci Technol 1997;15:619–26.
Atkin R, Craig VSJ, Wanless EJ, Biggs S. Mechanism of cationic surfactant adsorption at the solid-aqueous interface. Adv Colloid Interface Sci 2003;103:219–304.
Barati-Harooni A, Najafi-Marghmaleki A, Tatar A, Mohammadi AH. Experimental and modeling studies on adsorption of a nonionic surfactant on sandstone minerals in enhanced oil recovery process with surfactant flooding. J Mol Liq 2016;220:1022–32.
Belhaj AF, Elraies KA, Alnarabiji MS, Shuhli JABM, Mahmood SM, Ern LW. Experimental investigation of surfactant partitioning in Pre-CMC and Post-CMC regimes for enhanced oil recovery application. Energies 2019;12:1–15.
Bera A, Kumar T, Ojha K, Mandal A. Adsorption of surfactants on sand surface in enhanced oil recovery: Isotherms, kinetics and thermodynamic studies. Appl Surf Sci 2013;284:87–99.
Bera A, Mandal A, Belhaj H, Kumar T. Enhanced oil recovery by nonionic surfactants considering micellization, surface, and foaming properties. Pet Sci 2017;14:362–71.
Emadi S, Shadizadeh SR, Manshad AK, Rahimi AM, Mohammadi AH. Effect of nano silica particles on Interfacial Tension (IFT) and mobility control of natural surfactant (Cedr Extraction) solution in enhanced oil recovery process with nano - surfactant flooding. J Mol Liq 2017;248:163–7.
Eriksson T, Börjesson J, Tjerneld F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb Technol 2002;31:353–64.
Fatih Belhaj A, Abdalla Elraies K, Mohammad Mahmood S, Nazma Zulkifli N, Akbari S, Salaheldin Hussien O. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: a review. J Petrol Explor Prod Technol 2020;10:125–37.
Haq B, Liu J, Liu K, Shehri D Al. The role of biodegradable surfactant in microbial enhanced oil recovery. J Pet Sci Eng 2019.
Iglauer S, Wu Y, Shuler P, Tang Y, Goddard WA. New surfactant classes for enhanced oil recovery and their tertiary oil recovery potential. J Pet Sci Eng 2010;71:23–9.
Ijagbemi CO, Baek MH, Kim DS. Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J Hazard Mater 2009;166:538–46.
Juang LC, Wang CC, Lee CK. Adsorption of basic dyes onto MCM-41. Chemosphere 2006;64:1920–8.
Karnanda W, Benzagouta MS, AlQuraishi A, Amro MM. Effect of temperature, pressure, salinity, and surfactant concentration on IFT for surfactant flooding optimization. Arab J Geosci 2013;6:3535–44.
Lee SH, Roichman Y, Yi GR, Kim SH, Yang SM, Blaaderen A Van, et al. Characterizing and tracking single colloidal particles with video holographic microscopy. Opt Express 2007;15:18275–82.
Liu Y. New insights into pseudo-second-order kinetic equation for adsorption. Colloids Surfaces A Physicochem Eng Asp 2008;320:275–8.
Mandal A, Bera A, Ojha K, Kumar T. Characterization of surfactant stabilized nanoemulsion and its use in enhanced oil recovery. Soc Pet Eng - SPE Int Oilf Nanotechnol Conf 2012 2012:80–92.
Markandeya, Shukla SP, Dhiman N. Characterization and adsorption of disperse dyes from wastewater onto cenospheres activated carbon composites. Environ Earth Sci 2017;76:1–12.
Mi X, Li G, Zhu W, Liu L. Enhanced adsorption of orange II using cationic surfactant modified biochar pyrolyzed from cornstalk. J Chem 2016;2016:1–7.
Mohan K. Alkaline surfactant flooding for tight carbonate reservoirs. Proc - SPE Annu Tech Conf Exhib 2009;7:4727–38.
National Center for Biotechnology Information (NCBI). PubChem Compound Database; CID= 3423265 [Internet]. 2005 [cite 2020 Jan 13]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/3423265.
National Center for Biotechnology Information (NCBI). PubChem Compound Database; CID=5974 [Internet]. 2004 [cite 2020 Jan 13]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/5974.
Negin C, Ali S, Xie Q. Most common surfactants employed in chemical enhanced oil recovery. Petroleum 2017;3:197–211.
Nowrouzi I, Mohammadi AH, Manshad AK. Water-oil interfacial tension (IFT) reduction and wettability alteration in surfactant flooding process using extracted saponin from Anabasis Setifera plant. J Pet Sci Eng 2020.
Paria S, Khilar KC. A review on experimental studies of surfactant adsorption at the hydrophilic solid-water interface. Adv Colloid Interface Sci 2004;110:75–95.
Park S, Lee ES, Sulaiman WRW. Adsorption behaviors of surfactants for chemical flooding in enhanced oil recovery. J Ind Eng Chem 2015;21:1239–45.
Parnthong J, Kungsanant S, Chavadej S. The Influence of Nonionic Surfactant Adsorption on Enzymatic Hydrolysis of Oil Palm Fruit Bunch. Appl Biochem Biotechnol 2018;186:895–908.
Rabiu AM, Elias S, Oyekola O. Evaluation of Surfactant Synthesized from Waste Vegetable Oil to Enhance Oil Recovery from Petroleum Reservoirs. Energy Procedia 2016;100:188–92.
Rosen MJ. Surfactants and interfaccial phenomena. 2nd ed. USA: Wiley; 1989.
Rostami P, Mehraban MF, Sharifi M, Dejam M, Ayatollahi S. Effect of water salinity on oil/brine interfacial behaviour during low salinity waterflooding: A mechanistic study. Petroleum 2019;5:367–74.
Saxena N, Kumar S, Mandal A. Adsorption characteristics and kinetics of synthesized anionic surfactant and polymeric surfactant on sand surface for application in enhanced oil recovery. Asia-Pacific J Chem Eng 2018;13:11–4.
Seethepalli A, Adibhatla B, Mohanty KK. Physicochemical interactions during surfactant flooding of fractured carbonate reservoirs. SPE J 2004;9:411–8.
Sharma G, Mohanty KK. Wettability alteration in high-temperature and high-salinity carbonate reservoirs. SPE J 2013;18:646–55.
Shaw JE. Carboxylate surfactant systems exhibiting phase behavior suitable for enhanced oil recovery. J Am Oil Chem Soc 1984;61:1395–9.
Siddiqui MA, Kungsanant S, Chaiprapat S. Oil solubilization using surfactant for biohydrogen production. Adv Mater Res 2014;931–932:183–7.
Somasundaran P, Healy TW, Fuerstenau DW. Surfactant adsorption at the solid-liquid interface - Dependence of mechanism on chain length. J Phys Chem 1964;68:3562–6.
Spildo K, Sun L, Djurhuus K, Skauge A. A strategy for low cost, effective surfactant injection. J Pet Sci Eng 2014;117:8–14.
Tehrani-Bagha AR, Holmberg K. Solubilization of hydrophobic dyes in surfactant solutions. Materials (Basel) 2013;6:580–608.
Wetchakul W. Production of Palm Kernel-Diethanolamide in a Stirred-tank Reactor [master thesis]. Songkhla, Prince of Songkla University, 2013.
Wiśniewska M. The temperature effect on the adsorption mechanism of polyacrylamide on the silica surface and its stability. Appl Surf Sci 2012;258:3094–101.
Wo AJ, Bagaria HG, Yunshen C, Bryant SL, Jo KP. Nanoparticle stabilized carbon dioxide in water foams for enhanced oil recovery. SPE Improv Oil Recover Symp 2012;2:1496–502.