Removal of Iron from Groundwater by Ozonation: The Response Surface Methodology for Parameter Optimization 10.32526/ennrj/19/2020286
Main Article Content
Abstract
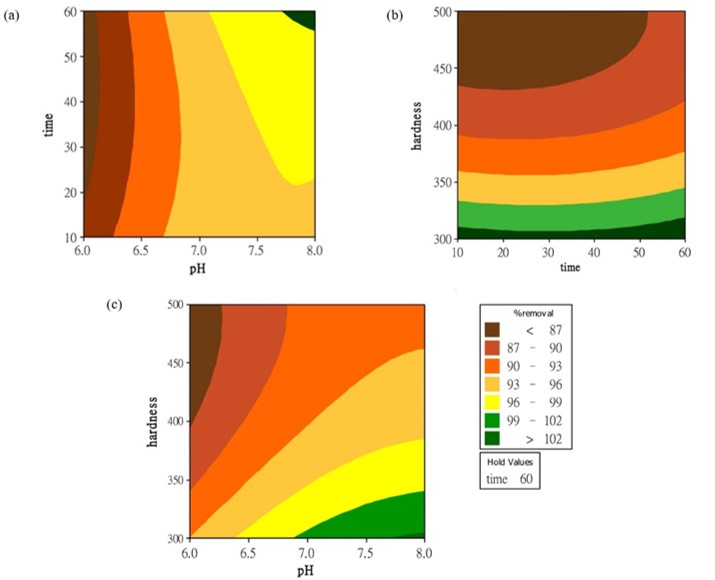
This research studied the possibility of using ozone to remove iron from groundwater. The optimum conditions were investigated using a Box-Behnken experiment design with statistical analysis by response surface technique. The three parameters investigated, pH (6.0-8.0), hardness (300-500 mg/L as CaCO3) and removal time (10 to 60 min) were independent parameters of iron removal. Data was examined for optimal conditions and included main effects and their interactions. Analysis of variance indicated that the proposed quadratic model successfully interpreted the experimental data with a coefficient of determination (R2) of 98.83% and adjusted R2 of 96.72%. Through this model, it could predict the iron removal efficiency under variable conditions. Furthermore, the optimum conditions were pH 6.99, hardness of 300 mg/L as CaCO3, and 10 min of reaction time. The predicted iron removal efficiency obtained from the model under the optimum conditions was 99.00%. The experiment confirmed that the optimum condition which validated the model’s accuracy of iron removal efficiency was 98.45%. The results showed that ozone can remove iron from groundwater.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Abraham N, James J, Banerji T, Menon R. Development of a novel groundwater iron removal system using adsorptive Fe(II) process. Groundwater for Sustainable Development 2020; 10:1-7.
Aljundi IH. Bromate formation during ozonation of drinking water: A response surface methodology study. Desalination 2011;277:24-8.
Araby R El, Hawash S, Diwani G El. Treatment of iron and manganese in simulated groundwater via ozone technology. Desalination 2009;249:1345-9.
Chaturvedi S, Dave PN. Removal of iron for safe drinking water. Desalination 2012;303:1-11.
Choothong N, Kaewvilai A, Laobuthee A, Lertworasirikul A. A novel colorimetric sensing material, poly(γ-glutamic acid)-graft-3, 4-dihydro-3-(2′-ethyl hydroxyl)-6-ethyl-1,3,2H-benzoxazine (γ-PGA-graft-ethyl-Bx), for iron(III) ions. Sains Malaysiana 2013;42(2):223-9.
Das B, Hazarika P, Saikia G, Kalita H, Goswami DC, Das HB, et al. Removal of iron from groundwater by ash: A systematic study of a traditional method. Journal of Hazardous Materials 2007;141:834-41.
Das B, Nandi BK. Removal of Fe (II) ions from drinking water using Electrocoagulation (EC) process: Parametric optimization and kinetic study. Journal of Environmental Chemical Engineering 2019;7:1-9.
Ellis D, Bouchard C, Lantagne G. Removal of iron and manganese from groundwater by oxidation and microfiltration. Desalination 2000;130:255-64.
Hachemi MEE, Naffrechoux E, Suptil J, Hausler R. Bicarbonate effect in the ozone-UV process in the presence of nitrate. Ozone: Science and Engineering 2013;35:3-7.
Kumar SS, Malyan SK, Kumar A, Bishnoi NR. Optimization of fenton’s oxidation by box Behnken design of response surface methodology for landfill leachate. Journal of Material and Environmental Science 2016;12:4456-66.
Pentamwa P, Thipthara W, Nuangon S. Removal of hardness from groundwater by synthetic resin from waste plastics. International Journal of Environmental Science and Development 2011;2(6):479-83.
Qiu P, Cui M, Kang K, Park B, Son Y, et al. Application of Box Behnken design with response surface methodology for modelling and optimizing ultrasonic oxidation of arsenite with H2O2. Central European Journal of Chemistry 2014;12(2):164-72.
Rice EW, Baird RB, Eaton AD. Standard Methods for the Examination of Water and Wastewater. 23rd ed. American Public Health Association, American Water Works Association, Water Environment Federation; 2017.
Sukmilin A, Boonchom B, Jarusuttirak C. Catalytic ozonation using iron-doped water treatment sludge as a catalyst for treatment of phenol in synthetic wastewater. Environment and Natural Resources Journal 2019;17(2):87-95.
Tayeb A, Tony MA, Mansour SA. Application of Box Behnken factorial design for parameters optimization of basic dye removal using nano hematile photo fenton. Applied Water Science 2018;8(138):1-9.
Thaweechai T, Kaewvilai A. Benzoxazine grafted poly(γ-glutamic acid) functional material: Synthesis, characterization and photophysical properties. Materials Chemistry and Physics 2019;227:117-22.
World Health Organization (WHO). World Health Organization Guidelines for Drinking Water Quality, Recommendations. Geneva, Switzerland: WHO; 1984. p. 79.