Characterization and Application of Mangosteen Peel Activated Carbon for Ammonia Gas Removal 10.32526/ennrj/19/2020298
Main Article Content
Abstract
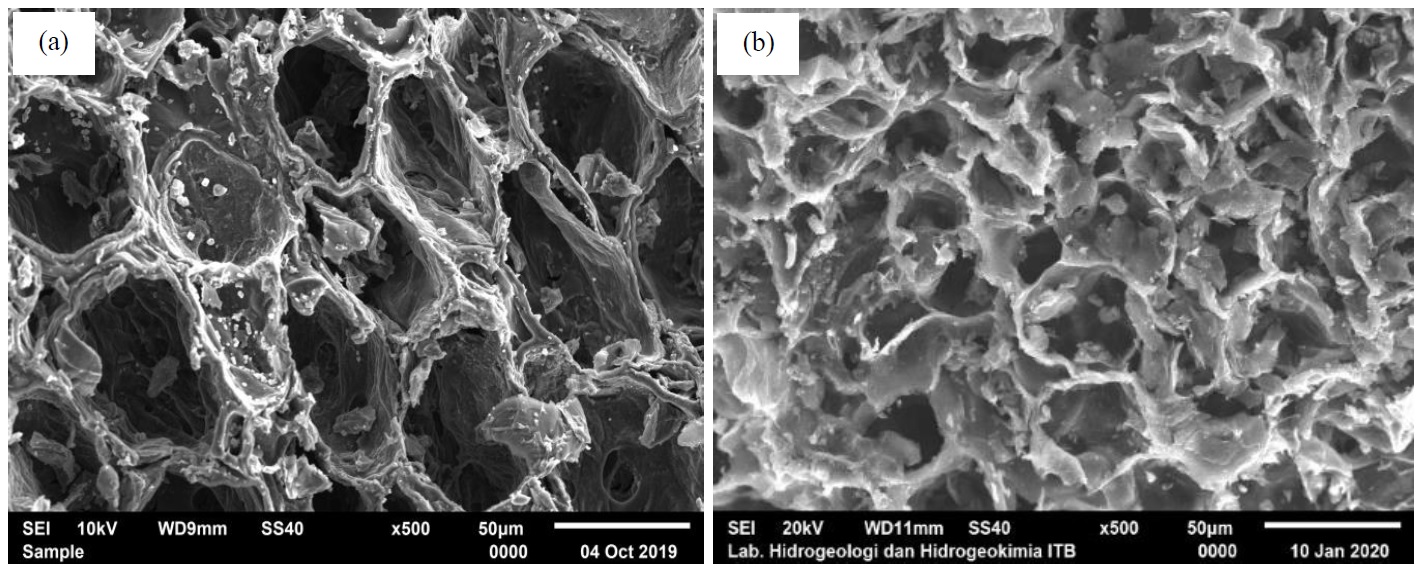
Mangosteen peel can be used as an activated carbon precursor because of its high lignin content and hardness. In this study, mangosteen peel activated carbon (MP-AC) was prepared by a physical activation method using CO2 at 850°C. The Brunauer-Emmett-Teller (BET) analysis was used to assess the optimal activation time to identify the largest surface area. The properties of MP-AC were characterized by the SEM-EDS and FTIR analyses. The results showed that MP-AC obtained from the 120-minute activation time had the largest BET specific surface area of 588.41 m2/g and was selected as an adsorbent in the dynamic adsorption of ammonia gas. The values of moisture content, ash content, and iodine number of MP-AC were 6.07%, 9.8%, and 1153.69 mg/g, respectively. Breakthrough curve indicated that with lower inlet concentration and higher adsorbent mass, longer breakthrough time is reached. Equilibrium data was best fitted to the Langmuir isotherm, while the pseudo-first order kinetic model favorably described the adsorption kinetics. The results revealed a potential to utilize MP-AC as an adsorbent for ammonia gas removal with average NH3 adsorption capacity of 0.41 mg/g.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Ahmad F, Daud WMAW, Ahmad MA, Radzi R, Azmi AA. The effects of CO2 activation, on porosity and surface functional group of cocoa (Theobroma cacao): Shell based activation carbon. Journal of Environmental Chemical Engineering 2013;1:378-88.
Ahmad MA, Alrozi R. Optimization of preparation conditions for mangosteen peel-based activated carbons for the removal of Remazol Brilliant Blue R using response surface methodology. Chemical Engineering Journal 2010;165:883-90.
Ahmad MA, Alrozi R. Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic, and thermodynamic studies. Chemical Engineering Journal 2011;171:510-6.
Ambroz F, Macdonald TJ, Martis V, Parkin IP. Evaluation of the BET theory for the characterization of meso and microporous MOFs. Small Methods 2018;2:1800173.
Basrur D, Bhat J. Preparation of activated carbon from mustard seed and its adsorption efficiency towards dye and acid. Journal of Urban and Environmental Engineering 2018; 12:266-76.
Bernal V, Giraldo L, Moreno-Piraján J. Physicochemical properties of activated carbon: Their effect on the adsorption of pharmaceutical compounds and adsorbate-adsorbent interactions. Journal of Carbon Research 2018;4:62.
Chandra TC, Mirna MM, Sunarso J, Sudaryanto Y, Ismadji S. Activated carbon from durian shell: Preparation and charavterization. Journal of the Taiwan Institute of Chemical Engineering 2009;40:457-62.
Chen Y, Huang B, Huang M, Cai B. On the preparation and characterization of activated carbon from mangosteen shell. Journal of the Taiwan Institute of Chemical Engineers 2011;42:837-42.
Cheremisinoff PN, Ellerbusch F. Carbon Adsorption Handbook. Michigan, USA: Ann Arbor Science Publishers; 1978.
Choo HS, Lau LC, Mohamed AR, Lee KT. Hydrogen sulfide adsorption by alkaline impregnated coconut shell ativated carbon. Journal of Engineering Science and Technology 2013;8:741-53.
Chung Y, Huang C, Liu CH, Bai H. Biotreatment of hydrogen sulfide- and ammonia-containing waste gases by fluidized bed bioreactor. Journal of the Air and Waste Management Association 2001;51:163-72.
Crini G, Lichtfouse E. Green adsorbents for Pollutant Removal: Innovative material. Heidelberg, Germany: Springer; 2018.
Devi AS, Latif PA, Tham YJ, Taufiq-Yap YH. Physical characterization of activated carbon derived from mangosteen peel. Asian Journal of Chemistry 2012;24:579-83.
Ding S, Liu Y. Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 2020;260:116382.
Domingo-Garcia M, Groszek AJ, Lopez-Garzon FJ, Perez-Mendora M. Dynamic adsorption of ammonia on activated carbons measured by flow microcalorimetry. Applied Catalysis A: General 2002;233:141-50.
El maguana Y, Elhadiri N, Benchanaa M, Chikri R. Adsorption thermodynamic and kinetic studies of methyl orange onto sugar scum powder as a low-cost inorganic adsorbent. Journal of Chemistry 2020;2020:1-10.
Foo KY, Hameed BH. Factors affecting the carbon yield and adsorption capability of the mangosteen peel activated carbon prepared by microwave assisted K2CO3 activation. Chemical Engineering Journal 2012;180:66-74.
Fernandez ME, Nunell GV, Bonelli PR, Cukierman AL. Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Industrial Crops and Products 2014;62:437-45.
Freundlich H. Over the adsorption in solution. Journal of Physics Chemistry 1906;57:385-470.
Gebreegziabher TB, Wang S, Nam H. Adsorption of H2S, NH3, and TMA from indoor air using porous corncob activated carbon: Isotherm and kinetic study. Journal of Environmental Chemical Engineering 2019;7:103234.
Ghasemi M, Ghasemi N, Zahedi G, Alwi SRW, Goodarzi M, Javadian H. Kinetic and equilibrium study of Ni(II) sorption from aqueous solutions onto Peganum harmala-L. International Journal of Environmental Science and Technology 2014;11:1835-44.
Giraldo L, Moreno-Piraján JC. CO2 adsorption on activated carbon prepared from mangosteen peel. Journal of Thermal Analysis and Calorimetry 2017;133:337-54.
Guo J, Xu WS, Chen YL, Lua AC. Adsorption of NH3 onto activated carbon prepared from palm shells impregnated with H2SO4. Journal of Colloid and Interface Science 2005; 281:285-90.
Hamzaoui M, Bestani B, Benderdouche N. The use of linear and nonlinear methods for adsorption isotherm optimization of basic green 4-dye onto sawdust-based activated carbon. Journal of Materials and Environmental Sciences 2018; 9:1110-8.
Hastuti N, Pari G, Setiawan D, Mahpudin M, Godang DM. Acidity and alkalinity level of Mayan bamboo activated charcoal (MBAC) on saturated vapor of acid chloride and natrium hydroxide. Widyariset 2015;1:41-50.
Ho YS, McKay G. Pseudo-second order model for sorption processes. Process Biochemistry 1999;34:451-65.
Kang DW, Ju SE, Kim DW, Kang M, Kim H, Hong CS. Emerging porous materials and their composites for NH3 gas removal. Advanced Science 2020;7:2002142.
Kutluay S, Baytar O, Şahin Ö. Equilibrium, kinetic and thermodynamic studies for dynamic adsorption of benzene in gas phase onto activated carbon produced from Elaeagnus angustifolia seeds. Journal of Environmental Chemical Engineering 2019;7:102947.
Lagergren S. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 1898;24:1-39.
Lan X, Jiang X, Song Y, Jing X, Xing X. The effect of activation temperature on structure and properties of blue coke-based activated carbon by CO2 activation. Green Processing and Synthesis 2019;8:837-45.
Langmuir I. The constitution and fundamental properties of solids and liquids. II. Liquids. Journal of the American Chemical Society 1917;3:1848-906.
Li Y, Wang X, Cao M. Three-dimensional porous carbon frameworks derived from mangosteen peel waste as promising materials for CO2 capture and supercapacitors. Journal of CO2 Utilization 2018;27:204-16.
Meneghetti E, Baroni P, Vieira RS, Da Silva MGC, Beppu MM. Dynamic adsorption of chromium ions onto natural and crosslinked chitosan membranes for wastewater treatment. Material Research 2010;13:89-94.
Mukti NIF, Prasetyo I, Mindaryani A. Preparation of ethylene adsorbent by pyrolysis of mangosteen peels. Proceeding of Indonesian National Seminar on Chemical Engineering 2015 Sustainable Energy and Mineral Processing for National Competitiveness; 2015 Oct 12-13; Gadjah Mada University Club Hotel, Yogyakarta: Indonesia; 2015.
Nasrullah A, Saad B, Bhat AH, Khan AS, Danish M, Isa MH, et al. Mangosteen peel waste as a sustainable precursor for high surface area mesoporous activated carbon: Characterization and application for methylene blue removal. Journal of Cleaner Production 2019;211:1190-200.
Nor NM, Lau LC, Lee KT, Mohamed AR. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control: A review. Journal of Environmental Chemical Engineering 2013;1:658-66.
Nowicki P, Kazmierczak J, Pietrzak R. Comparison of physicochemical and sorption properties of activated carbons prepared by physical and chemical activation of cherry stones. Powder Technology 2015;269:312-9.
Patel H. Fixed bed column adsorption study: A comperehensive review. Applied Water Science 2019;9:1-17.
Rangabhashiyam S, Balasubramanian P. The potential of lignocellulosic biomas precursors for biochar production: Performance, mechanism, and wastewater application. Industrial Crops and Products 2019;128:405-23.
Rattanapan S, Pengjam P, Kongsune P. Preparation and characterization of mangosteen peel activated carbon. Thaksin University Journal 2014;17:13-21.
Reza MS, Yun CS, Afroze S, Radenahmad N, Bakar MSA, Saidur R, et al. Preparation of activated carbon from biomass and its’ applications in water and gas purification: A review. Arab Journal of Basic and Applied Sciences 2020;27:208-38.
Ro KS, Lima IM, Reddy GB, Jackson MA, Gao B. Removing gaseous NH3 using biochar as an adsorbent. Agriculture 2015;5:991-1002.
Saka C. BET, TG-DTG, FT-IR, SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2. Journal of Analytical and Applied Pyrolysis 2012;95:21-4.
Saputro EA, Wulan VDR, Winata BY, Yogaswara RR, Erliyanti NK. The process of activated carbon from coconut shells through chemical activation. Journal of Science and Technology 2020;9:23-9.
Statistics Indonesia (BPS). Statistics of Annual Fruit and Vegetable Plants Indonesia 2018. Jakarta, Indonesia: BPS-Statistics Indonesia; 2019.
Unugul T, Nigiz FU. Preparation and characterization an active carbon adsorbent from waste mandarin peel and determination of adsorption behavior on removal of synthetic dye solutions. Water, Air and Soil Pollution 2020;231:538.
Xu R, Tian H, Pan S, Prior SA, Feng Y, Batchelor WD, et al. Global ammonia emissions from synthetic nitrogen fertilizer applications in agricultural systems: Empirical and process-based estimates and uncertainty. Global Change Biology 2019;25:314-26.
Vohra M. Treatment of gaseous ammonia emissions using date palm pits based granular activated carbon. International Journal of Environmental Research and Public Health 2020;17:1519.
Yang T, Lua AC. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. Journal of Colloid and Interface Science 2003;267:408-17.
Yani M, Nurcahyani PR, Rahayuningsih M. Ammonia removal by biofilter technique packed with coral and granulated activated carbon (GAC) inoculated with enriched nitrifying bacteria. Journal of Agroindustrial Technology 2013;23:22-9.
Yeom C, Kim Y. Adsorption of ammonia using mesoporous alumina prepared by a templating method. Environmental Engineering Research 2017;22:401-6.
Yuliusman, Nasruddin, Afdhol MK, Haris F, Amiliana RA, Hanafi A, et al. Production of activated carbon from coffee grounds using chemical and physical activation method. Advanced Science Letters 2017;23:5751-5.