Natural Phosphates Characterization and Evaluation of their Removal Efficiency of Methylene Blue and Methyl Orange from Aqueous Media 10.32526/ennrj/20/202100147
Main Article Content
Abstract
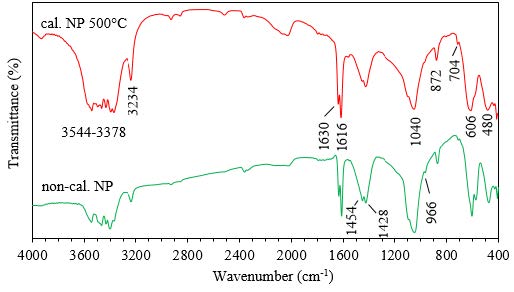
This study evaluated the capacity of a rock phosphate for the adsorption of organic dyes methylene blue MB and methyl orange MO in aqueous solution, in order to minimize the impact of these dyes on the environment. The physicochemical characterization of natural phosphates (NP) shows that its mineralogy is carbonate-fluorapatite, calcite and quartz, as demonstrated by X-ray diffraction. An infrared (IR) analysis completed the structural study by confirming the characteristic bands of a carbonated fluorapatite type B. The influence of adsorbent dose, pH, initial concentration and temperature of the dye solution on adsorption onto NP was studied. The experimental results show that MB is adsorbed almost entirely at an adsorbent dose of 1 g/L and at a more basic pH and that the Langmuir model describes its isotherm well. For MO, adsorption is performed at acidic pH, such that discoloration reaches 60% at pH 4 and NP adsorbent dose of 10 g/L. The maximum adsorbed amounts of MB (pH=9) and MO (pH=4) were found to be 9.54 and 1.09 mg/g, respectively. The kinetic data were analyzed to show that the pseudo-second-order model seems to be the most appropriate to describe the adsorption dynamics of both dyes on the naturel phosphate. The thermodynamic results show that the adsorption is endothermic for MB and exothermic for MO. So, rock phosphate shows a good potential as a sorbent for cationic dyes removal from wastewater.
Article Details
Published articles are under the copyright of the Environment and Natural Resources Journal effective when the article is accepted for publication thus granting Environment and Natural Resources Journal all rights for the work so that both parties may be protected from the consequences of unauthorized use. Partially or totally publication of an article elsewhere is possible only after the consent from the editors.
References
Al-Ghouti M, Khraisheh MAM, Ahmad MNM, Allen S. Thermodynamic behaviour and the effect of temperature on the removal of dyes from aqueous solution using modified diatomite: A kinetic study. Journal of Colloid and Interface Science 2005;287:6-13.
Aines RD, Rossman GR. Water in minerals? A peak in the infrared. Journal of Geophysical Research: Solid Earth 1984;89:4059-71.
Assimeddine M, Abdennouri M, Barka N, Rifi EH, Sadiq M. Physicochemical characterization of Moroccan natural clays and the study of their adsorption capacity for the methyl orange and methylene blue removal from aqueous solution. Journal of Environmental Treatment Techniques 2020; 8(4):1258-67.
Barka N, Assabbane A, Nounah A, Laanab L, Aît-Ichou Y. Removal of textile dyes from aqueous solutions by natural phosphate as a new adsorbent. Desalination 2009;235:264-75.
Bedin KC, de Azevedo SP, Leandro PKT, Cazetta AL, Almeida VC. Bone char prepared by CO2 atmosphere: Preparation optimization and adsorption studies of Remazol Brilliant Blue R. Journal of Cleaner Production 2017;161:288-98.
Bensalah H, Bekheet MF, Younssi SA, Ouammou M, Gurlo A. Removal of cationic and anionic textile dyes with Moroccan natural phosphate. Journal of Environmental Chemical Engineering 2017;5(3):2189-99.
Boughzala K, Bouzouita K. Synthesis and characterization of strontium-calcium-lanthanum apatites Sr7-xCaxLa3(PO4)3(SiO4)3 F2 0≤x≤2. Comptes Rendus Chimie 2015;18(8):858-66.
Chen BY. Toxicity assessment of aromatic amines to Pseudomonas luteola: Chemostat pulse technique and dose-response analysis. Process Biochemistry 2006;41(7):1529-38.
Combes RD, Haveland-Smith RB. A review of the genotoxicity of food, drug and cosmetic colors and other azo, triphenylmethane and xanthene dyes. Mutation Research/Reviews in Genetic Toxicology 1982;98(2):101-248.
Dakkach M, Atlamsani A, Sebti S. Natural phosphate modified by vanadium: A new catalyst for oxidation of cycloalkanones and α-ketols with oxygen molecular. Comptes Rendus Chimie 2012;15(6):482-92.
Damodar RA, Jagannathan K, Swaminathan T. Decolourization of reactive dyes by thin film immobilized surface photoreactor using solar irradiation. Solar Energy 2007;81(1):1-7.
Daneshvar N, Salari D, Khataee AR. Photocatalytic degradation of azo dye acid red 14 in water: Investigation of the effect of operational parameters. Journal of Photochemistry and Photobiology A: Chemistry 2003;157(1):111-6.
Deniz F, Saygideger SD. Investigation of adsorption characteristics of Basic Red 46 onto gypsum: Equilibrium, kinetic and thermodynamic studies. Desalination 2010;262 (1-3):161-5.
Drouet C. Apatite formation: Why it may not work as planned, and how to conclusively identify apatite compounds. BioMed Research International 2013;2013(4):1-12.
Elmoubarki R, Mahjoubi FZ, Elhalil A, Tounsadi H, Abdennouri M, Sadiq M, et al. Ni/Fe and Mg/Fe layered double hydroxides and their calcined derivatives: Preparation, characterization and application on textile dyes removal. Journal of Materials Research and Technology 2017;6(3):271-83.
Elmoubarki R, Mahjoubi FZ, Tounsadi H, Moustadraf J, Abdennouri M, Zouhri A, et al. Adsorption of textile dyes on raw and decanted Moroccan clays: Kinetics, equilibrium and thermodynamics. Water Resources and Industry 2015;9:16-29.
Fahami A, Nasiri-Tabrizi B. Mechanochemical behavior of CaCO3-P2O5-CaF2 system to produce carbonated fluorapatite nanopowder. Ceramics International 2014;40(9):14939-46.
Farnane M, Tounsadi H, Machrouhi A, Elhalil E, Mahjoubi FZ, Sadiq M, et al. Dye removal from aqueous solution by raw maize corncob and H3PO4 activated maize corncob. Journal of Water Reuse and Desalination 2018;8(2):214-24.
Fleet ME. Infrared spectra of carbonate apatites: ν2-region bands. Biomaterials 2009;30(8):1473-81.
Fleet ME, Liu X. Accommodation of the carbonate ion in fluorapatite synthesized at high pressure. American Mineralogist 2008;93:1460-9.
Freundlich H, Heller W. The adsorption of cis- and trans-azobenzene. Journal of the American Chemical Society 1939;61:2228-30.
Freundlich HMF. Over the adsorption in solution. The Journal of Physical Chemistry 1906;57:385-471.
Furuzono T, Walsh D, Sato K, Sonoda K, Tanaka J. Effect of reaction temperature on the morphology and size of hydroxyapatite nanoparticles in an emulsion system. Journal of Materials Science Letters 2001;20(2):111-4.
Gallala W, Herchi F, Ben Ali I, Abbassi L, Gaied ME, Montacer M. Beneficiation of phosphate solid coarse waste from redayef (Gafsa Mining Basin) by grinding and flotation techniques. Procedia Engineering 2016;138:85-94.
Giles CH, MacEwan TH, Nakhwa SN, Smith D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. Journal of the Chemical Society 1960;846:3973-93.
Guo Y, Zhu Z, Qiu Y, Zhao J. Enhanced adsorption of acid brown 14 dye on calcined Mg/Fe layered double hydroxide with memory effect. Chemical Engineering Journal 2013;219:69-77.
Gupta K, Khatri OP. Fast and efficient adsorptive removal of organic dyes and active pharmaceutical ingredient by microporous carbon: Effect of molecular size and charge. Chemical Engineering Journal 2019;378:Article No.122218.
Heiss GS, Gowan B, Dabbs ER. Cloning of DNA from a Rhodococcus strain conferring the ability to decolorize sulfonated azo dyes. FEMS Microbiology Letters 1992; 99:221-6.
Ho YS, Mckay FG. Kinetic models for the sorption of dye from aqueous solution by wood. Process Safety and Environmental Protection 1998;76:183-91.
Kenzour A, Belhouchet H, Kolli M, Djouallah S, Kherifi D, Ramesh S. Sintering behavior of anorthite-based composite ceramics produced from natural phosphate and kaolin. Ceramics International 2019;45:20258-65.
Kuang Y, Zhang X, Zhou S. Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 2020;12(2):587-605.
Lagergren S. About the theory of so-called adsorption of soluble substance. Kungliga Svenska Ventenskapsakademiens Handlingar 1898;24:1-39.
Langmuir I. The constitution and fundamental properties of solids and liquids. Part I. Solids. Journal of the American Chemical Society 1916;38:2221-95.
Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society 1918;40:1361-403.
Machrouhi A, Boumya W, Khnifira M, Sadiq M, Abdennouri M, Elhalil A, et al. Synthetic dyes adsorption and discoloration of a textile wastewater effluent by H3PO4 and H3BO3 activated Thapsia transtagana biomass. Desalination and Water Treatment 2020;202:435-49.
Madupalli H, Pavan B, Tecklenburg MMJ. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. Journal of Solid-State Chemistry 2017;255:27-35.
Mahjoubi FZ, Khalidi A, Abdennouri M, Barka N. M-Al-SO4 layered double hydroxides (M=Zn, Mg, or Ni): Synthesis, characterization and textile dyes removal efficiency. Desalination and Water Treatment 2016;57:21564-76.
Mgaidi A, Ben Brahim F, Oulahna D, Nzihou A, El Maaoui M. Chemical and structural changes of raw phosphate during heat treatment. High Temperature Materials and Processes 2004;23(3):185-94.
Mohammadi N, Khani H, Gupta VK, Amereh E, Agarwal S. Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies. Journal of Colloid and Interface Science 2011;362:457-62.
Mouflih M, Aklil A, Jahroud N, Gourai M, Sebti S. Removal of lead from aqueous solutions by natural phosphate. Hydrometallurgy 2006;81:219-25.
Nikcevic I, Jokanovic V, Mitric M, Nedic Z, Makovec D, Uskokovic D. Mechanochemical synthesis of nanostructured fluorapatite/fluorhydroxyapatite and carbonated fluorapatite/ fluorhydroxyapatite. Journal of Solid State Chemistry 2004; 177:2565-74.
Pignatello JJ. The measurement and interpretation of sorption and desorption rates for organic compounds in soil media. Advances in Agronomy 1999;69:1-73.
Rytwo G, Ruiz-Hitzky E. Enthalpies of adsorption of methylene blue and crystal violet to montmorillonite: Enthalpies of adsorption of dyes to montmorillonite. Journal of Thermal Analysis and Calorimetry 2003;71:751-9.
Sabar S, Abdul-Aziz H, Yusof NH, Subramaniam S, Foo KY, Wilson LD, et al. Preparation of sulfonated chitosan for enhanced adsorption of methylene blue from aqueous solution. Reactive and Functional Polymers 2020;151:Article No.104584.
Sadiq M, Abdennouri M, Barka N, Baalala M, Lamonier C, Bensitel M. Influence of the crystal phase of magnesium phosphates catalysts on the skeletal isomerization of 3,3-dimethylbut-1-ene. Canadian Chemical Transactions 2015; 3(2):225-33.
Tóth J. Thermodynamical correctness of gas/solid adsorption isotherm equations. Journal of Colloid and Interface Science 1994;163:299-302.
Tsuda S, Matsusaka N, Madarame H, Ueno S, Susa N, Ishida K, et al. The comet assay in eight mouse organs: Results with 24 azo compounds. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2000;465(1-2):11-26.
Wang M, Li G, Huang L, Xue J, Liu Q, Bao N, et al. Study of ciprofloxacin adsorption and regeneration of activated carbon prepared from Enteromorpha prolifera impregnated with H3PO4 and sodium benzenesulfonate. Ecotoxicology and Environmental Safety 2017;139:36-42.
Wang M, Qian R, Bao M, Gu C, Zhu P. Raman, FT-IR and XRD study of bovine bone mineral and carbonated apatites with different carbonate levels. Materials Letters 2018;210:203-6.
Yous R, Mohellebi F, Cherifi H, Amrane A. Competitive biosorption of heavy metals from aqueous solutions onto Streptomyces rimosus. Korean Journal of Chemical Engineering 2018;35:890-9.
Zahrani EM, Fathi MH. The effect of high-energy ball milling parameters on the preparation and characterization of fluorapatite nanocrystalline powder. Ceramics International 2009;35:2311-23.