Fabrication, Characterization, and Biocompatibility Study of Gelatin-Blended Fibroin Scaffold
Main Article Content
Abstract
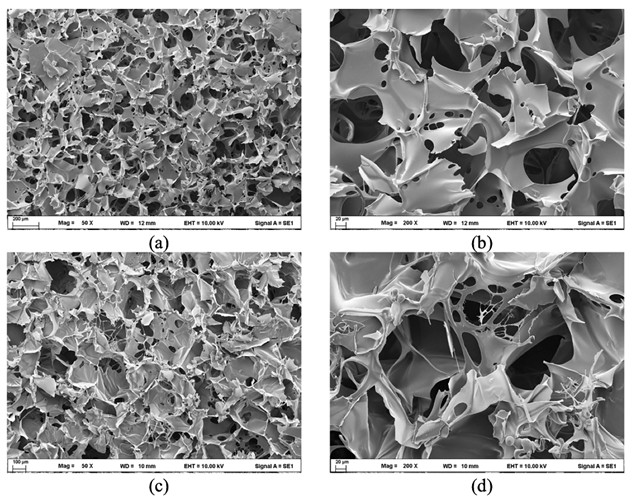
This research investigates the biocompatibility of gelatin-blended fibroin scaffold fabricated by freeze-dry process. The morphology showed interconnected spongy spheres and polygons shapes with a pore size of 14.144 ± 11.31 and 19.4822 ± 18.71 micrometers, respectively. The formula ratio of fibroin: gelatin of 8:2 contained more random coil, while the formula ratio of fibroin: gelatin of 7:3 exhibited more β-sheet structure. The result revealed that gelatin influences fibroin conformational transition from random coil to beta, which might result from better hydrophobic characteristics. The surface of the formula ratio of fibroin: gelatin at 8:2 and 7:3 was 125.88 ± 9.85 and 130.07 ± 3.72 degrees, indicating the formula ratio of fibroin: gelatin at 7:3 had more hydrophobicity than the formula ratio of fibroin: gelatin at 8:2. The biocompatibility of scaffolds was determined for both formulas of human keratinocyte (HaCaT) and African green monkey kidney (Vero) cell lines by MTT assay. The formula ratio of fibroin: gelatin at 8:2 with various concentrations of 62.50, 125, 250, 500, and 1,000 µg/mL had cell viability percentages of 96.31, 101.74, 97.81, 104.15, and 103.22%, respectively. The formula ratio of fibroin: gelatin at 7:3 with various concentrations of 62.50, 125, 250, 500, and 1,000 µg/mL had cell viability percentages of 106.38, 104.28, 108.54, 97.42, and 97.65%, respectively. The growth of cell lines showed better performance with the hydrophilic characteristics of the scaffold, and it did not make it toxic to the HaCaT and Vero cell lines. These results demonstrated the possibility of gelatin-blended scaffold as a supporting material for skin tissue engineering, especially for wound healing.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
O'Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Materials Today, 2011, 14(3), 88-95.
Chamchongkaset, J.; Kanokpanont, S.; Kaplan, D.L.; Damrongsakkul, S. Modification of Thai Silk Fibroin Scaffolds by Gelatin Conjugation for Tissue Engineering. Advanced Materials Research, 2008, 55, 685-688.
Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials Fabrication from Bombyx mori Silk Fibroin. Nature Protocols, 2011, 6, 1612-1631. https://doi.org/10.1038/nprot.2011.379.
Luangbudnark, W.; Viyoch, J.; Laupattarakasem, W.; Surakunprapha, P.; Laupattarakasem, P. Properties and Biocompatibility of Chitosan and Silk Fibroin Blend Films for Application in Skin Tissue Engineering. The Scientific World Journal, 2012, 697201. https://doi.org/10.1100/2012/697201.
Duangpakdee, A.; Laomeephol, C.; Jindatip, D.; Thongnuek, P.; Ratanavaraporn, J.; Damrongsakkul, S. Crosslinked Silk Fibroin/Gelatin/Hyaluronan Blends as Scaffolds for Cell-Based Tissue Engineering. Molecules, 2021, 26(11), 3191. https://doi.org/10.3390/ molecules26113191.
Cole, C.G.B. Gelatin. In Encyclopedia of Food Science and Technology, 2nd ed; Frederick, F.J., Ed.; John Wiley & Sons, New York, 2000; 1183-1188.
Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk Fibroin/Gelatin Microcarriers as Scaffolds for Bone Tissue Engineering. Materials Science and Engineering: C, 2020, 106, 110116. https://doi.org/10.1016/ j.msec.2019.110116.
Surya, I.; Waesateh, K.; Masa, A.; Hayeemasae, N. Potency of Urea-Treated Halloysite Nanotubes for the Simultaneous Boosting of Mechanical Properties and Crystallization of Epoxidized Natural Rubber Composites. Polymers, 2021, 13, 3068. https://doi.org/10.3390/polym13183068.
Wang, J.; Yang, Q.; Cheng, N.; Tao, X.; Zhanga, Z.; Sun, X.; Zhang, Q. Collagen/Silk Fibroin Composite Scaffold Incorporated with PLGA Microsphere for Cartilage Repair. Materials Science and Engineering. C, Materials for Biological Applications, 2016, 61, 705-711.
Ghafari, R.; Jonoobi, M.; Amirabad, L.M.; Oksman, K.; Taheri, A.R. Fabrication and Characterization of Novel Bilayer Scaffold from Nanocellulose Based Aerogel for Skin Tissue Engineering Applications. International Journal of Biological Macromolecules, 2019, 136, 796-803.
Chonanant, C. Fabrication the Biomaterial Scaffold for Tissue Engineering by Freeze-Drying Technique. Journal of Medical Technology and physical therapy, 2019, 31(3), 370-381.
Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of Material Surfaces in Regulating Bone and Cartilage Cell Response. Biomaterials, 1996, 17(2), 137-146.
Martin, C.; Subathra, R.; Jayanthi, V.; Reddy, S.; Varghese, J.; Rela, M.; Kalkura, N. Collagen Rich Scaffolds for Liver Engineering: a Step Forward to an Articicial Liver. Journal of Clinical and Experimental Hepatology, 2016, 6, S86–S87.
Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining Information about Protein Secondary Structures in Aqueous Solution using Fourier Transform IR Spectroscopy. Nature Protocols, 2015, 10(3), 382-396.
Nagai, T.; Suzuki, N.; Tanoue, Y.; Kai, N. Functional Property of Honey from Echium vulgare. Food and Nutrition Sciences, 2012, 3(8), 614-620.
Silva Júnior, Z.S.; Botta, S.B.; Ana, P.A.; Franca, C.M.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Deana, A.; Bussadori, S.K. Effect of Papain-Based Gel on Type I Collagen-Spectroscopy Applied for Microstructural Analysis. Scientific. Reports, 2015, 5, 11448. https://doi.org/10.1038/srep11448.
Chadefaux, C.; Le Hô, A.-S.; Bellot-Gurlet, L.; Reiche, I. Curve-Fitting Microe-ATR-FRIR Studies of the Amide I and II Bands of Type I Collagen in Archaeological Bone Materials. e-PRESERVATION Science, 2009, 6, 129-137.
Efraim, Y.; Schoen, B.; Zahran, S.; Davidov, T.; Vasilyev, G.; Baruch, L.; Zussman, E.; Machluf, M. 3D Structure and Processing Methods Direct the Biological Attributes of ECM-Based Cardiac Scaffolds. Scientific Reports, 2019, 9, 5578. https://doi.org/10.1038/s41598-019-41831-9.
Yeelack, W. Hybrid Biomimetic Scaffold of Silk Figroin/Collaget Type I Films for Tissue Engineering: Preparation and Characterization. M.Sc. thesis, Prince of Songkla University, Songkla, 2014.
Paxton, N.; Woodruff, M. Measuring Contact Angles on Hydrophilic Porous Scaffolds by Implementing a Novel Raised Platform Approach: A technical note. Polymer Advanced Technologies, 2022, 33, 3759-3765.
Gil, E.S.; Frankowski, D.J.; Bowman, M.K.; Gozen, A.O.; Hudson, S.M.; Spontakm, R.J. Mixed Protein Blends Composed of Gelatin and Bombyx mori Silk Fibroin: Effects of Solvent-Induced Crystallization and Composition. Biomacromolecules, 2006, 7, 728-735.
Asakura, T.; Kuzuhara, A.; Tabeta, R.; Saito, H. Conformational Characterization of Bombyx mori Silk Fibroin in the Solid State by High-Frequency Carbon-13 Cross Polarization-Magic Angle Spinning NMR, X-ray Diffraction, and Infrared Spectroscopy. Macromolecules, 1985, 18, 1841-1845.
Kim, U.J.; Parka, J.; Kima, H.J.; Wada, M.; Kaplan, D.L. Three-Dimensional Aqueous-Derived Biomaterial Scaffolds from Silk Fibroin. Biomaterials, 2005, 26, 2775-2785.