Induction of Gynogenetic Diploids the Tropical Oyster, Crassostrea belcheri 1873 (Ostreids: Ostreoidea)
Main Article Content
Abstract
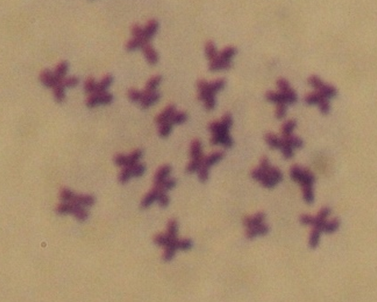
The induction of diploid gynogenesis in the Tropical oyster, Crassostrea belcheri 1873, was carried out through two main experiments: 1) the destruction of spermatozoa DNA using ultraviolet (UV) irradiation, and 2) the induction of gynogenetic diploids. UV light source was placed 30 cm over the sperm for various durations, including 0 (control), 30, 60, 90, and 120 seconds. The study revealed that the highest survival rate was observed in trochophore at 90 minutes (42.5 ± 2.50%) compared to the normal control C. belcheri (p>0.05). Based on cytogenetic study, haploid (n=10), diploid (2n=20), and aneuploid were observed in all trials, while only diploid (2n=20) could be seen in the control group. Diploid gynogenesis was induced using 100 µmol 6-DMAP for 10 minutes. The study found that the survival rate and developmental stages in both trochophore and D-shape were gradually decreasing as the duration of UV rays increased. The highest survival rate of trochophore in the control group was 75.20 ± 4.84%, while survival rates in other experimental groups ranged from 52.22 ± 4.82% to 59.33 ± 13.57%. However, regarding the survival rate of D-shape larvae, their survival rates were relatively low across all trials. They displayed abnormal embryo development, distorted shape, unnatural swimming, and were smaller than normal (40-50 µm). Moreover, haploid (n=10), diploid (2n=20), triploid (3n=30), tetraploid (4n=40), and aneuploid were observed in all trials, while only diploid (2n=20) could be discovered in the control group. However, gynogenesis in this tropical oyster is still limited at this time. Therefore, suitable conditions for inducing gynogenesis in oysters should be further developed.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Yuan, TC.; Liang, Y.; Cai, WR.; Hai, YR.; Ping, WZ. Triploid of Crassostrea gigas induced with 6 dimethylaminopurine: blocking polar body I, J. Fish. China. 1999, 2, 128-132.
Guo, X.; Hershberger, WK.; Cooper, K.; Chew, KK. Artificial gynogenesis with ultraviolet light-irradiated sperm in the Pacific oyster, Crassostrea gigas. I. Induction and survival. Aquaculture. 1993, 113, 201-214.
Manan, H.; Hidayati, AN.; Lyana, NA.; Amin-Safwan, A.; Ma, H.; Kasan, NA.; Ikhwanuddin, M. A review of gynogenesis manipulation in aquatic animals. Aquaculture and Fisheries. 2022, 7(1), 1-6.
Li Q, Kijima A. Segregation of microsatellite alleles in gynogenetic diploid pacific abalone (Haliotis discus hannai). Marine Biotechnology. 2005, 7, 669-676.
Gosling, E. Bivalve molluscs: biology, ecology and culture: John Wiley & Sons. 2008.
Teeraya, C.; Natthapong, T.; Siwa, T. The season of Oyster maturation in Bandon Bay, Suratthani Province. Technical Paper No. 2/2006. Coastal Fisheries Research and Development Bureau, Department of Fisheries, Ministry of Agriculture and Cooperatives. 2006. 21p. (In Thai).
Galtsoff, PS. The American oyster Crassostrea virginica (Gmelin). US Fish Wildlf. Serv. Fish. Bull. 1964, 64: 1-480.
Zhang, Z.; Wang, Y.; Zhang, Z. Induction of gynogenetic diploids in the small abalone, Hauotis diversicolor supertexta. J. Shellfish Res. 2004, 23(4), 1115-1122.
Fujino K, Arai K, Iwadare K, Yoshida T, Nakajima S. Induction of gynogenetic diploid by inhibiting second meiosis in the Pacific abalone (Haliotis discus hannai). Bull. Jpn. Soc. Sci. Fish. 1990, 56: 1755-1763.
Pan, Y.; Li, Q.; Yu, R.; Wang, R. Induction of gynogenetic diploids and cytological studies in the zhikong scallop, Chlamys farreri. Aquatic Living Resources. 2004, 17, 201-206.
Fairbrother, JE. Viable gynogenetic diploid Mytilus edulis (L.) larvae produced by ultraviolet light irradiation and cytochalasin B shock. Aquaculture. 1994, 126(1-2), 25-34.
Scarpa, J.; Komaru, A, Wada KT. Gynogenetic induction in the mussel, Mytilus galloprovincialis. Bull. Natl. Res. Inst. Aquac. (Jpn.). 1994, 23, 33-41.
Guo, X.; Allen, S. Sex determination and polyploid gigantism in the dwarf surfclam (Mulinia lateralis Say). Genetics. 1994, 138(4), 1199-1206.
Helm, M.; Bourne, MN.; Lovatelli, A. Hatchery culture of bivalves: a practical manual. 2004.
Nowland, SJ.; O’Connor, WA.; Elizur, A.; Southgate, PC. Evaluating spawning induction methods for the tropical black-lip rock oyster, Saccostrea echinate. Aquaculture Reports. 2021, 20, 100676.
Chooseangjaew, S.; Tanyaros, S.; Koedprang, W.; Tanomtong, A. Triploid Induction by the use of 6- dimethylaminopurine (6-DMAP) for the Tropical Oyster, Crassostrea belcheri (Sowerby, 1871). Proceedings of the 9th Rajamangala University of Technology International Conference (9th RMUTIC). 2018, 180-188.
Thomas, P.; Mallia, JV.; Muthiah, P. Induction of triploidy in Indian edible oyster Crassostrea madrasensis (Preston) using 6-Dimethylaminopurine. Asian Fish Sci. 2006, 19(1), 15-20.
Kijima, A. Effect of irradiation on genetic inactivation of sperm using marketing Tissue culture petri-dish in the Pacific abalone Haliotis discus hannai, Tohoku J. Agric. Res. 1992, 42, 73-81.
LI, Q.; Osada, M.; Kashihara, M.; Hirohashi, K.; Kijima, A. Induction of gynogenetic diploids and cytological studies in the Pacific oyster, Crassostrea gigas. Aquaculture Science. 2000, 48(2), 185-191.
Li, Q.; Osada, M.; Kashihara, M.; Hirohashi, K.; Kijima, A. Artificially Induced gynogenetic diploid in the Pacific abalone, Haliotis discus hannai. Fish Genet. Breed. Sci. 1999, 28, 85-94.