Smartphone-Based Spectrophotometer for Facile and Fast Determination of Lipid Peroxidation in Local Fried Food
Main Article Content
Abstract
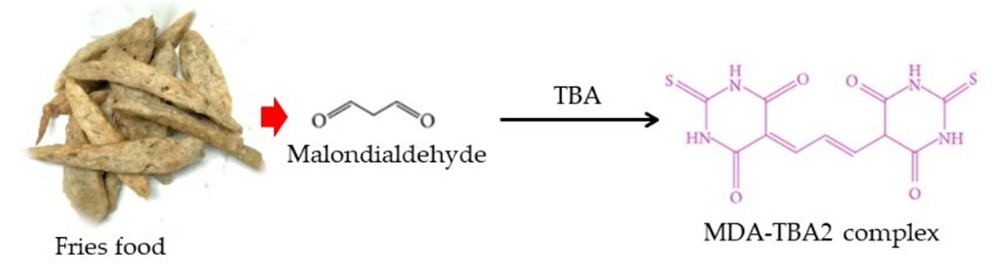
During lipid peroxidation in foods, deterioration rancidity occurred, and a toxic by-product also accumulated. The well-known marker of lipid peroxidation in food is malondialdehyde (MDA), suspected to be carcinogenic and mutagenic in humans. MDA level is determined using thiobarbituric acid (TBA) assay. The pink color of the MDA-TBA2 complex after the reaction can be measured spectrophotometrically at 530-540 nm. Several analytical methods, including smartphone-based methods, have been used to determine the MDA-TBA2 complex, such as UV-Vis spectrophotometry and HPLC-DAD. Therefore, this research aimed to determine lipid peroxidation in fried food using a simple smartphone-based spectrophotometer. The device was established using a paper box, LED lamps, and a test tube. Various concentrations of MDA were reacted with TBA reagent and then submitted to the device. RGB intensity data were converted to absorbance values and used to construct linear regression. Results showed that the G value from the smartphone-based spectrophotometer provided consistent results with R2 of 0.9869, including 0.93 and 95.17% precision and accuracy, respectively. Then, the developed device was finally used to determine the MDA in local fried food samples. The concentration of MDA in fried foods was successfully determined with high precision (0.96) and accuracy (88.33 %) compared to the traditional UV-Vis spectrophotometric method. Thus, this study provides a practical guideline for developing quick and easy accessibility, portability, and low-cost spectrophotometer for lipid peroxidation assessment in fried food and other future food matrices.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Bordin, K.; Kunitake, M. T.; Aracava, K. K.; Trindade, C. S. Changes in food caused by deep fat frying – A review. Archivos Latinoamericanos de Nutrición. 2013, 63(1), 5-13. ISSN 0004-0622.
Wang, D.; Xiao, H.; Lyu, X.; Chen, H.; Wei, F. Lipid oxidation in food science and nutritional health: A comprehensive review. Oil Crop Science. 2023; 8(1), 35-44. https://doi.org/10.1016/j.ocsci.2023.02.002.
Ahmed, M.; Pickova, J.; Ahmad, T.; Liaquat, M.; Farid, A.; Jahangir, M. Oxidation of lipids in foods. Sarhad Journal of Agriculture. 2016, 32(3), 230-238. http://dx.doi.org/10.17582/journal.sja/2016.32.3.230.238.
Ma, L.; He, Q.; Qiu, Y.; Liu, H.; Wu, J.; Liu, G.; Brennan, C.; Brennan, M. A.; Zhu, L. Food matrixes play a key role in the distribution of contaminants of lipid origin: A case study of malondialdehyde formation in vegetable oils during deep-frying. Food Chemistry. 2021, 347, 129080. https://doi.org/10.1016/j.foodchem.2021.129080.
Velasco, J.; Dobarganes, C.; Márquez-Ruiz, G. Oxidative rancidity in foods and food quality. Chemical Deterioration and Physical Instability of Food and Beverages. 2010, 5, 3-32. https://doi.org/10.1533/9781845699260.1.3.
Papastergiadis, A.; Mubiru, E.; Van Langenhove, H.; De Meulenaer, B. Malondialdehyde measurement in oxidized foods: evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. Journal of agricultural and food chemistry. 2012, 60(38), 9589–9594. https://doi.org/10.1021/jf302451c.
Całyniuk, B.; Grochowska-Niedworok, E.; Walkiewicz, K. W.; Kawecka, S.; Popiołek, E.; Fatyga, E. Malondialdehyde (MDA) – product of lipid peroxidation as marker of homeostasis disorders and aging. Annales Academiae Medicae Silesiensis. 2016, 70, 224-8. https://doi.org/10.18794/aams/65697
Ghani, Md. A.; Barril, C.; Bedgood, D. R.; Prenzler, P. D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chemistry. 2017, 230, 195-207. https://doi.org/10.1016/j.foodchem.2017.02.127.
Aguilar Diaz De Leon, J.; Borges, C. R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. Journal of visualized experiments. 2020, 159, e61122. https://doi.org/10.3791/61122.
Zeb, A.; Ullah, F. A simple spectrophotometric method for the determination of TBARS in fried fast foods. Journal of Analytical Methods in Chemistry, 2016; 4, 9412767. https://doi.org/10.1155/2016/9412767.
Fashi, A.; Cheraghi, M.; Badiee, H.; Zamani, A. An analytical strategy based on the combination of ultrasound assisted flat membrane liquid phase microextraction and a smartphone reader for trace determination of malondialdehyde. Talanta. 2020, 209, 120618. https://doi.org/10.1016/j.talanta.2019.120618.
Weitner, T.; Inić, S.; Jablan, J.; Gabričević, M.; Domijan, A. Spectrophotometric determination of malondialdehyde in urine suitable for epidemiological studies. Croatica Chemica Acta. 2016, 89(1), 133–139. https://doi.org/10.5562/cca2902.
Mendes, R.; Cardoso, C.; Pestana, C. Measurement of malondialdehyde in fish: A comparison study between HPLC methods and the traditional spectrophotometric test. Food Chemistry. 2009, 112(4), 1038-1045. https://doi.org/10.1016/j.foodchem.2008.06.052.
Hussain, I.; Bowden, A. K. Smartphone-based optical spectroscopic platforms for biomedical applications: a review. Biomedical Optics Express. 2021, 12(4), 1974-1998. https://doi.org/10.1364/BOE.416753.
Mohamed, A. A.; Shalaby, A. A. Digital imaging devices as sensors for iron determination. Food Chemistry. 2019, 274, 360-367. https://doi.org/10.1016/j.foodchem.2018.09.014.
Hlaing, W. M. M.; Kruanetr, S.; Ruengsitagoon, W. RGB colorimetric method for the quantitative analysis of levocetirizine tablets. Isan Journal of Pharmaceutical Sciences. 2020, 16(3), 65-75. https://doi.org/10.14456/ijps.2020.19.
Moonrungsee, N.; Prachain, C.; Bumrungkij, C.; Peamaroon, N.; Jakmunee, J. A simple device with a smartphone camera for determination of salicylic acid in foods, drugs and cosmetics. The Journal of King Mongkut's University of Technology North Bangkok, 2018; 28(3), 639-648. https://doi.org/10.14416/j.kmutnb.2018.03.001.
Stéfani, I. E. A.; Marcelo, B. L.; Inakã, S. B.; Wellington, S. L.; Luciano, F. A.; Mário, C. U. A.; Edvan, C. S. A digital image-based flow-batch analyzer for determining Al(III) and Cr(VI) in water. Microchemical Journal. 2013, 109, 106-111. https://doi.org/10.1016/j.microc.2012.03.029.
Fauziah, P. N.; Maskoen, A. M.; Yuliati, T.; Widiarsih, E. Optimized steps in determination of malondialdehyde (MDA) standards on diagnostic of lipid peroxidation. Padjadjaran Journal of Dentistry. 2018, 30(2), 136-139. http://dx.doi.org/10.24198/pjd.vol30no2.18329.
Lindon, J. C.; Tranter, G. E.; Koppenaal, D. W. Encyclopedia of spectroscopy and spectrometry. 3rd ed.; Academic Press. 2010, pp 451-455.
Oshina, I.; Spigulis, J. Beer-Lambert law for optical tissue diagnostics: current state of the art and the main limitations. Journal of biomedical optics. 2021, 26(10), 100901. https://doi.org/10.1117/1.JBO.26.10.100901.
Mohamed, O.; Shantier, S.; Abureid, I.; Gadkariem, E. Development of an HPLC method for the simultaneous Determination of Levodopa and Carbidopa in Pharmaceutical dosage forms. Hacettepe University Journal of the Faculty of Pharmacy. 2021, 41(4), 221-227. https://doi.org/10.52794/hujpharm.1006726.
Rahman, H.M.; Rahman, M.M. Estimation of limit of detection (LOD), limit of quantification (LOQ) and machine standardization by Gas Chromatography. Annals of Bangladesh Agriculture. 2015, 19(2), 55-65. ISSN1025-482X.
Martysiak-Żurowska, D.; Stołyhwo, A. Content of malondialdehyde (MDA) in infant formulae and follow-on formulae. Polish Journal of Food and Nutrition Sciences. 2006, 56(3), 323–328.
Pandey, M. C.; Harilal, P. T.; Radhakrishna, K. Effect of processing conditions on physico-chemical and textural properties of shami kebab. International Food Research Journal. 2014, 21(1), 223-228.