Antiproliferative Activity and GC-MS Analysis from the Leaves Extract of Different Cultivars Carica Papaya
Main Article Content
Abstract
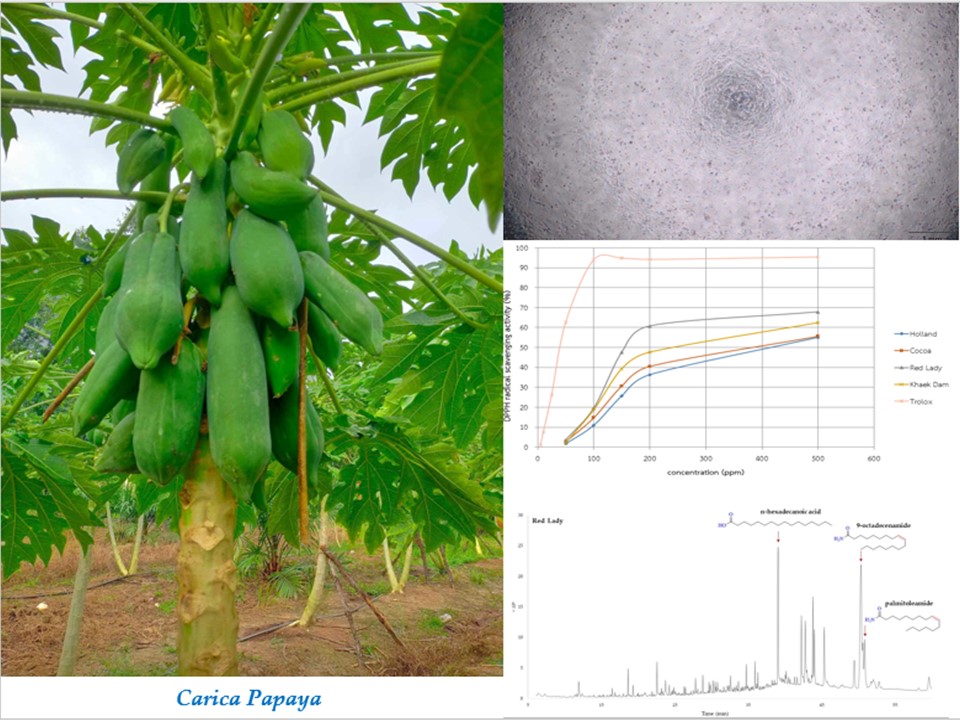
Papaya is the fruit of the Carica papaya plant.Several secondary metabolites from the Caricagenus have been reported to exhibit interesting biological activities.Its leaves are normally considered discarded.The research aimed to examine the antiproliferativeand antioxidanteffects of Carica papayaleaves from four different cultivars-Cocoa, Holland, Khaek Dam, and Red Ladyand evaluate the chemical composition of the extracts through GC−MS analysis. The MTT assay evaluated the antiproliferative activity of all extracts.Red Lady exhibited higher effectiveness against MCF-7, SW620, and Vero cell lines comparedto Khaek Dam, with IC50values of 90.88 ± 0.39, 258.45 ± 2.16, and 301.73 ± 0.73 μg/mL, respectively.Cocoa and Holland extracts showed no cytotoxic effects on the mentioned cell lines. Antioxidant activity, measured through DPPH radical scavenging assays, revealed that Red Lady had the highest antioxidant capacity (IC50163.87 μg/mL), followed by Khaek Dam, Cocoa, and Holland. As a result, the GC−MS analysis concentrated on the extracts of Lady and Khaek Dam. The chromatograms revealed that the extractsfrom Red Lady displayed 23 components, while those from Khaek Dam contained 22. The primary metabolite produced in Khaek Dam were n-hexadecanoic acid (17.53%), 1-heptadecanecarboxylicacid (6.86%), and loliolide (5.58%),while in Red Lady 9-octadecenamide(20.82%), n-hexadecanoicacid (8.26%), palmitoleamide (5.43%)were produced. This indicates a difference in the chemical composition between the two cultivars. It is clear from this study that the chosen species includeda range of potent phytochemicalswith antiproliferative characteristics.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Hewitt, H.; Whittle, S.; Lopez, S.; Bailey, E.; Weaver, S. Topical use of papaya in chronic skin ulcer therapy in Jamaica. West. Indian. Med. J. 2000, 49, 32–33.
Lim, T. Edible Medicinal and Non-Medicinal Plants: Volume 1, Fruits, Springer Science+Business Media. New York. 2012, 693–717.
Krishna, K.L.; Paridhavi, M.; Patel, J.A. Review on nutritional, medicinal and pharmacological properties of papaya (Carica papaya Linn.). Nat. Prod. Rad. 2008, 7, 364–373.
Vij, T.; Prashar Y. A review on medicinal properties of Carica papaya Linn. Asian. Pac. J. Trop. Dis. 2015, 5(1), 1–6. https://doi.org/10.1016/s2222-1808(14)60617-4
Dharmarathna, S.L.C.A.; Wickramasinghe, S.; Waduge, R.N.; Rajapakse, R.P.V.J.; Kularatne, S.A.M. Does Carica papaya leaf-extract increase the platelet count? An experimental study in a murine model. Asian. Pac. J. Trop. Biomed. 2013, 3(9), 720–724. https://doi.10.1016/S2221-1691(13)60145-8
Sharma, A.; Sharma, R.; Sharma, M.; Kumar, M.; Barbhai, M.D.; Lorenzo, J.M.; Sharma, S.; Samota, M.K.; Atanassova, M.; Caruso, G.; Naushad, M.; Radha Chandran, D.; Prakash, P.; Hasan, M.; Rais, N.; Dey, A.; Mahato, D.K.; Dhumal, S.; Singh, S.; Senapathy, M.; Rajalingam, S.; Visvanathan, M.; Saleena, L.A.K.; Mekhemar, M. Carica papaya L. leaves: Deciphering its antioxidant bioactives, biological activities, innovative products, and safety aspects. Oxid. Med. Cell. Longev. 2022, Article ID 2451733 https://doi.org/10.1155/2022/2451733
Teng, W.C.; Chan, W.; Suwanarusk, R.; Ong, A.; Ho, H.K.; Russell, B.; Rénia, L.; Koh, H.L. In vitro antimalarial evaluations and cytotoxicity investigations of Carica papaya leaves and Carpaine. Nat. Prod. Commun. 2019, 14(1). https://doi.10.1177/1934578X1901400110
Singh, S.P.; Kumar, S.; Mathan, S.V.; Tomar, M.S.; Singh, R.K.; Verma, P.K.; Kumar, A.; Kumar, S.; Singh, R.P.; Acharya, A. Therapeutic application of Carica papaya leaf extract in the management of human diseases. Daru. 2020, 28(2), 735–744. https://doi.10.1007/s40199-020-00348-7.
Nugroho, A.; Heryani, H.; Choi, J.S.; Park, H.J. Identification and quantification of flavonoids in Carica papaya leaf and peroxynitrite-scavenging activity. Asian. Pac. J. Trop. Biomed. 2017, 7(3), 208–213. https://doi.org/10.1016/s2222-1808(14)60617-4
Santana, L.F.; Inada, A.C.; Espirito Santo, B.L.S.D.; Filiú, W.F.O.; Pott, A.; Alves, F.M.; Guimarães, R.C.A.; Freitas, K.C.; Hiane, P.A. Nutraceutical potential of Carica papaya in metabolic syndrome. Nutrients. 2019, 11(7), 1608. https://doi.org/10.3390/nu11071608
Agarwal, A.; Vyas, S.; Agarwal, D.P. Therapeutic benefits of Carica papaya leaf extracts in dengue fever patients. Sch. J. Appl. Med. Sci. 2016, 4(2A), 299–302. https://doi.10.36347/sjams.2016.v04i02.003
Meenakshi, S.; Umayaparvathi, S.; Arumugam, M.; Balasubramanian, T. In vitro antioxidant properties of FTIR analysis of two sea weeds of Gulf of Mannar. Asian. Pac. J. Trop. Biomed. 2011, 1, S66–70. https://doi.org/10.1016/S2221-1691(11)60126-3
Jaiswal, P.; Kumar, P.; Singh, V.K.; Singh, D.K. Carica papaya Linn: A potential source for various health problems. J. Pharm. Res. 2010, 3, 998–1003.
Wu, Y.Y.; Li, W.; Xu, Y.; Jin, E.H.; Tu. Y.Y. Evaluation of the antioxidant effects of four main theaflavin derivatives through chemiluminescence and DNA damage analyses. J. Zhejiang. Univ. Sci. B. 2011, 12, 744–751. https://doi.org/10.1631/jzus.b1100041
Chaithada, P.; Whenngean, P.; Fungfueng, R.; Maungchanburee, S. Correlation between total flavonoid content and total phenolic content on antioxidant activity of ethanol extracts from three cultivars of papaya leaves. Int. J. Res. Pharm. Sci. 2020, 11(2), 1883–1887. http://doi.org/10.26452/ijrps.v11i2.2099
Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Schumacher, A.M. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. 1972, 3, 59–61.
Eldahshan, O.A. Rhoifolin: A potent antiproliferative effect on cancer cell lines. Br. J. Pharm. Res. 2013, 3, 46–53. https://doi.org/10.9734/BJPR/2013/1864
Wasman, S.Q.; Mahmood, A.A.; Chua, L.S.; Alshawsh, M.A.; Hamdan, S. Antioxidant and gastroprotective activities of Andrographis paniculata (Hempedu Bumi) in Sprague Dawley rats. Indian J. Exp. Biol. 2011, 49(10), 767–772.
Palafox-Carlos, H.; Yahia, E.; Islas-Osuna, M.A.; Gutierrez-Martinez, P.; Robles-Sánchez, M.; González-Aguilar, G.A. Effect of ripeness stage of mango fruit (Mangifera indica L., cv. Ataulfo) on physiological parameters and antioxidant activity. Scientia Horticulturae. 2012, 135, 7–13.
Yemis, O.; Bakkalbasi, E.; Artik, N. Antioxidative activities of grape (Vitis vinifera) seed extracts obtained from different varieties grown in Turkey. International Journal of Food Science & Technology. 2008, 43(1), 154–159. http://doi.10.1111/j.1365-2621.2006.01415.x
Nisa, F.Z.; Astuti, M.; Haryana, S.M.; Murdiati, A. Antioxidant activity and total flavonoid of Carica Papaya L. leaves with different varieties, maturity and solvent. Agritech. 2019, 39, 54–59, http://doi.10.22146/agritech.12813
Zhang, R.; Lv, J.; Yu, J.; Xiong, H.; Chen, P.; Cao, H.; John Martin, J.J. Antioxidant analysis of different parts of several cultivars of Papaya (Carica Papaya L.). Int. J. Fruit Sci. 2022, 22(1), 438–452. https://doi.org/10.1080/15538362.2022.2047138
Gorane, A.; Naik, A.; Nikam, T.; Tripathi, T.; Ade, A. GC-MS analysis of phytocomponents of C. papaya variety red lady. J. Pharmacogn. Phytochem. 2018, 7(2), 553–555.
Al-Seadi, H.L.; Sabti, M.Z.; Taain, D.A. GC-MS Analysis of Papaya Leaf Extract (Carica Papaya L.) .1 IOP Conf. Ser.: Earth Environ. Sci. 2021, 910 012011. https://doi.10.1088/1755-1315/910/1/012011
Smrati, S.; Verma, O.; Kumar, R. Phytochemical profiling, GC-MS analysis and antioxidant capacity of extracts of Carica papaya leaves. IJBPAS. 2022, 11(9), 4069–4079.
Ano, Y.; Ozawa, M.; Kutsukake, T.; Sugiyama, S.; Uchida, K.; Yoshida, A.; Nakayama, H. Preventive effects of a fermented dairy product against Alzheimer's disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. Plos. One. 2015, 10(3), e0118512. https://doi.org/10.1371/journal.pone.0118512
Olaoluwa, O.; Moronkola, D.; Taiwo, O.; Iganboh, P. Volatile oil composition, antioxidant and antimicrobial properties of Boerhavia erecta L. and Euphorbia hirta L. Trends. Phytochem. Res. 2018, 2, 171–178.
El-Moez, S.I.A.; Abdelmonem, M..; Gomaa, A.M.; Aziz, M.F.A. In vitro antibacterial activities of dietary medicinal ethanolic extracts against pathogenic reference strains of animal origin. Afr. J. Microbiol. Res. 2013, 7, 5261–5270. https://doi.org/10.5897/AJMR2013.5477.
Wisitpongpun, P.; Suphrom, N.; Potup, P.; Nuengchamnong, N.; Calder, P.C.; Usuwanthim, K. In vitro bioassay-guided identification of anticancer properties from Moringa oleifera Lam. leaf against the MDA-MB-231 Cell Line. Pharmaceuticals (Basel). 2020, 13(12), 464. https://doi.10.3390/ph13120464.
Sangpairoj, K.; Settacomkul, R.; Siangcham, T.; Meemon, K.; Niamnont, N.; Sornkaew, N.; Tamtin, M.; Sobhon, P.; Vivithanaporn, P. Hexadecanoic acid-enriched extract of Halymenia durvillei induces apoptotic and autophagic death of human triple-negative breast cancer cells by upregulating ER stress. Asian. Pac. J. Trop. Biomed. 2022, 12(3), 132–140. https://doi.10.4103/2221-1691.338922
Nazarudin, M.F.; Isha, A.; Mastuki, S.N.; Ain, N.M.; Mohd Ikhsan, N.F.; Abidin, A.Z; Aliyu-Paiko, M. Chemical composition and evaluation of the alpha-glucosidase inhibitory and cytotoxic properties of marine algae ulva intestinalis, Halimeda macroloba, and Sargassum ilicifolium. Evid. Based. Complement. Alternat. Med. 2020, 2020, 2753945. https://doi.10.1155/2020/2753945.
Ravi, L.; Krishnan, K. Cytotoxic potential of n-hexadecanoic acid extracted from Kigelia pinnata leaves. Asian J. Cell Biol. 2017, 12, 20–27. https://doi.10.3923/ajcb.2017.20.27
Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22(5), 2587–2590
Bharath, B.; Perinbam, K.; Devanesan, S.; AlSalhi, M. S.; Saravanan, M. Evaluation of the anticancer potential of hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J. Mol. Struct. 2021, 1235, 130229. https://doi.10.1016/j.molstruc.2021.130229
Dra, L.A.; Brahim, M.A.S.; Boualy, B.; Aghraz, A.; Barakate, M.; Oubaassine, S.; Markouk, M.; Larhsini, M. Chemical composition, antioxidant and evidence antimicrobial synergistic effects of Periploca laevigata essential oil with conventional antibiotics. Ind. Crops. Prod. 2017, 109, 746–752. https://doi.org/10.1016/j.indcrop.2017.09.028
Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug. Des. 2012, 80(3), 434–439. https://doi.org/10.1111/j.1747-0285.2012.01418.x.
Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. https://orcid.org/0000-0002-1013-6089
Capó, X.; Martorell, M.; Tur, J.A.; Sureda, A.; Pons, A. 5-Dodecanolide, a compound isolated from Pig Lard, presents powerful anti-inflammatory properties. Molecules. 2021, 26, 7363. https://doi.org/10.3390/molecules26237363
Rahbar, N.; Shafaghat, A.; Salimi, F. Antimicrobial activity and constituents of the hexane extracts from leaf and stem of Origanum vulgare L. ssp. Viride (Boiss.) Hayek. growing wild in Northwest Iran. J. Med.Plants Res. 2012, 6(13), 2681–2685. https://doi.org/10.5897/JMPR11.1768
Zhenga, C.J.; Yooa, J.S.; Leeb, T.G.; Choc, H.Y.; Kimd, Y.H.; Kim, W.G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Letters. 2005, 5157–5162. https://doi.10.1016/j.febslet.2005.08.028.
Grabarczyk, M.; Wińska, K.; Mączka, W.; Potaniec, B.; Anioł, M. Loliolide-the most ubiquitous lactone. Folia Biol. Oecologica. 2015, 11, 1–8. https://doi.org/10.1515/fobio-2015-0001
Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Kim, H.S.; Fernando, I.P.S.; Ahn, G. (−)-Loliolide isolated from Sargassum Horneri protects against fine dust-induced oxidative stress in human keratinocytes. Antioxidants. 2020, 9, 474. https://doi.org/10.3390/antiox9060474.
Li, L.L.; Zhao, H.H.; Kong, C.H. (−)-Loliolide, the most ubiquitous lactone, is involved in barnyardgrass-induced rice allelopathy. J. Exp. Bot. 2020, 71, 1540–1550. https://doi.org/10.1093/jxb/erz497.