Biodegrading Lignocellulosic Agricultural Waste using Phanerochaete chrysosporium and Electrical Current Stimulation
Main Article Content
Abstract
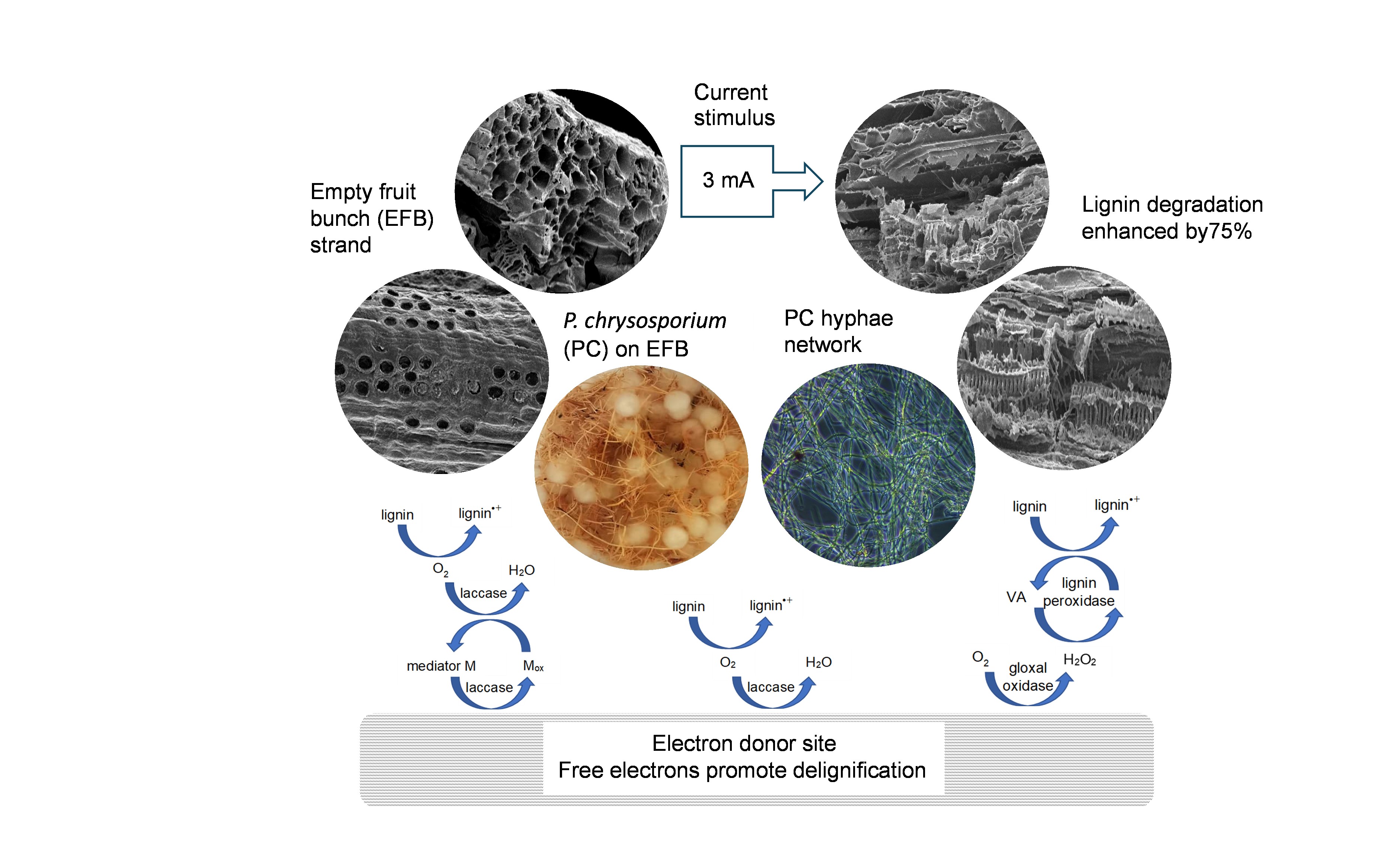
White-rot fungi (WRF), such as Phanerochaete chrysosporium, play a significant role in the lignin degradation (LD) of biomass, an essential process in the carbon recycling of terrestrial ecosystems. However, the rapid development of the agroindustry has imposed a daunting task on biomass waste management. One green initiative focuses on enhancing the bioremediation of lignin since it forms a resistant barrier to chemical and biological LD. This work demonstrated that electric current stimulation (ECS) can markedly enhance LD by P. chrysosporium. Palm oil empty fruit bunches (EFBs) were utilized as a lignin-rich substrate for P. chrysosporium. These were placed in a 250-ml enclosure filled with unbuffered potato dextrose broth (PDB) as the electrolyte. The ECS was supplied in situ in two ways: (1) by inserting a zinc anode/air electrode redox couple into the enclosure to produce a self-sustaining discharge current (DC), and (2) by connecting the enclosure to an external current (EC). The lignin content (LC) of the EFBs was assessed after 30 days of exposure to fungal microbes in an uncontrolled environment. The fungal LD rate was highest at 3 mA and even doubled under the influence of the EC, enhancing the lignin removal by 74.6%. The proposed method is much simpler and cheaper than the electrocatalytic reactions produced by the electro-Fenton method.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Rahayu, D. E.; Wirjodirdjo, B.; Hadi, W. Availability of Empty Fruit Bunch as Biomass Feedstock for Sustainability of Bioenergy Product (System Dynamic Approach). AIP Conf. Proc., 2019, 2194 (December). https://doi.org/10.1063/1.5139827.
Mohammad, I. N.; Ongkudon, C. M.; Misson, M. Physicochemical Properties and Lignin Degradation of Thermal-Pretreated Oil Palm Empty Fruit Bunch. Energies, 2020, 13(22). https://doi.org/10.3390/en13225966.
Eriksson, K.-E. L.; Robert A. Blanchette; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components; T. E. Timell, Ed.; Springer Berlin Heidelberg, 1990. https://doi.org/10.1016/0300-9629(91)90156-7.
Shen, J.; Hou, L.; Sun, H.; Hu, M.; Zang, L.; Zhang, Z.; Ji, D.; Zhang, F. Effect of an Electro-Fenton Process on the Biodegradation of Lignin by Trametes Versicolor. BioResources, 2020, 15(4), 8039–8050. https://doi.org/10.15376/biores.15.4.8039-8050.
Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J. M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric Analysis as a New Method to Determine the Lignocellulosic Composition of Biomass. Biomass and Bioenergy, 2011, 35 (1), 298–307. https://doi.org/10.1016/j.biombioe.2010.08.067.
Christopher, L. P.; Yao, B.; Ji, Y. Lignin Biodegradation with Laccase-Mediator Systems. Front. Energy Res., 2014, 2 (MAR), 1–13. https://doi.org/10.3389/fenrg.2014.00012.
Singh, D.; Chen, S. The White-Rot Fungus Phanerochaete Chrysosporium: Conditions for the Production of Lignin-Degrading Enzymes. Appl. Microbiol. Biotechnol., 2008, 81(3), 399–417. https://doi.org/10.1007/s00253-008-1706-9.
Sukri, A.; Othman, R.; Abd-Wahab, F.; Noor, N. M. Self-Sustaining Bioelectrochemical Cell from Fungal Degradation of Lignin-Rich Agrowaste. Energies, 2021, 14 (8). https://doi.org/10.3390/en14082098.
Rosli, N. S.; Harun, S.; Jahim, J. M.; Othaman, R. Chemical and Physical Characterization of Oil Palm Empty Fruit Bunch. Malaysian J. Anal. Sci., 2017, 21 (1), 188–196. https://doi.org/10.17576/mjas-2017-2101-22.
Vasileva, E.; Parvanova-Mancheva, T.; Beschkov, V.; Alexieva, Z.; Gerginova, M.; Peneva, N. Effects of Constant Electric Field on Biodegradation of Phenol by Free and Immobilized Cells of Bradyrhizobium Japonicum 273. ChemEngineering, 2021, 5(4). https://doi.org/10.3390/chemengineering5040075.
Lovley, D. R.; Fraga, J. L.; Blunt-Harris, E. L.; Hayes, L. A.; Phillips, E. J. P.; Coates, J. D. Humic Substances as a Mediator for Microbially Catalyzed Metal Reduction. Acta Hydrochim. Hydrobiol., 1998, 26(3), 152–157. https://doi.org/10.1002/(SICI)1521-401X(199805)26:3<152::AID-AHEH152>3.0.CO;2-D
Yadav, M.; Vivekanand, V. Chaetomium Globosporum: A Novel Laccase Producing Fungus for Improving the Hydrolyzability of Lignocellulosic Biomass. Heliyon, 2019, 5(3), e01353. https://doi.org/10.1016/j.heliyon.2019.e01353.
Abdel-Hamid, A. M.; Solbiati, J. O.; Cann, I. K. O. Insights into Lignin Degradation and Its Potential Industrial Applications. Adv. Appl. Microbiol., 2013, 82, 1–28. https://doi.org/10.1016/B978-0-12-407679-2.00001-6.
González-Ramírez, D.; Muro-Urista, C.; Arana-Cuenca, A.; Téllez-Jurado, A.; González-Becerra, A. Enzyme Production by Immobilized Phanerochaete Chrysosporium Using Airlift Reactor. Biologia (Bratisl)., 2014, 69(11), 1464–1471. https://doi.org/10.2478/s11756-014-0453-x.
Lee, H.; Jang, Y.; Choi, Y. S.; Kim, M. J.; Lee, J.; Lee, H.; Hong, J. H.; Lee, Y. M.; Kim, G. H.; Kim, J. J. Biotechnological Procedures to Select White Rot Fungi for the Degradation of PAHs. J. Microbiol. Methods, 2014, 97(1), 56–62. https://doi.org/10.1016/j.mimet.2013.12.007.
Smith, M.; Thurston, C. F.; Wood, D. A. Fungal Laccase: Role in Delignification and Possible Industrial Application. In Multi-Cpper Oxidases; World Scientific, 1997; pp 201–224.
Brenelli, L.; Squina, F. M.; Felby, C.; Cannella, D. Laccase-Derived Lignin Compounds Boost Cellulose Oxidative Enzymes AA9. Biotechnol. Biofuels, 2018, 11 (1), 1–12. https://doi.org/10.1186/s13068-017-0985-8.
Shamsudin, S.; Md Shah, U. K.; Zainudin, H.; Abd-Aziz, S.; Mustapa Kamal, S. M.; Shirai, Y.; Hassan, M. A. Effect of Steam Pretreatment on Oil Palm Empty Fruit Bunch for the Production of Sugars. Biomass and Bioenergy, 2012, 36, 280–288. https://doi.org/10.1016/j.biombioe.2011.10.040.
Galiwango, E.; Rahman, N. S. A.; Al-Marzouqi, A. H.; Abu-Omar, M. M.; Khaleel, A. A. Klason Method: An Effective Method for Isolation of Lignin Fractions from Date Palm Biomass Waste. Chem. Process Eng. Res., 2018, 57(0), 46–58.
Beschkov, V.; Alexieva, Z.; Parvanova-Mancheva, T.; Vasileva, E.; Gerginova, M.; Peneva, N.; Stoyanova, K. Phenol Biodegradation by the Strain Pseudomonas Putida Affected by Constant Electric Field. Int. J. Environ. Sci. Technol., 2019, 17(4), 1929–1936. https://doi.org/10.1007/s13762-019-02591-1.
Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczyński, A. Lignin Degradation: Microorganisms, Enzymes Involved, Genomes Analysis and Evolution. FEMS Microbiol. Rev., 2017, 41(6), 941–962. https://doi.org/10.1093/femsre/fux049.
Bourbonnais, R.; Paice, M. G.; Reid, I. D.; Lanthier, P.; Yaguchi, M. Lignin Oxidation by Laccase Isozymes from Trametes Versicolor and Role of the Mediator 2,2’-Azinobis(3-Ethylbenzthiazoline-6-Sulfonate) in Kraft Lignin Depolymerization. Appl. Environ. Microbiol., 1995, 61(5), 1876–1880. https://doi.org/10.1128/aem.61.5.1876-1880.1995.
Martínez, Á. T.; Speranza, M.; Ruiz-Dueñas, F. J.; Ferreira, P.; Camarero, S.; Guillén, F.; Martínez, M. J.; Gutiérrez, A.; Del Río, J. C. Biodegradation of Lignocellulosics: Microbial, Chemical, and Enzymatic Aspects of the Fungal Attack of Lignin. Int. Microbiol., 2005, 8(3), 195–204.
Schoemaker, H. E.; Harvey, P. J.; Bowen, R. M.; Palmer, J. M. On the Mechanism of Enzymatic Lignin Breakdown. FEBS Lett., 1985, 183(1), 7–12. https://doi.org/10.1016/0014-5793(85)80942-X.
Lange, H.; Decina, S.; Crestini, C. Oxidative Upgrade of Lignin - Recent Routes Reviewed. Eur. Polym. J., 2013, 49(6), 1151–1173. https://doi.org/10.1016/j.eurpolymj.2013.03.002.