Induction of Mutation in Toothbrush Orchids using Ethylmethane Sulphonate (EMS) and Detection of Genetic Variation by SSR (Simple Sequence Repeat) Marker
Main Article Content
Abstract
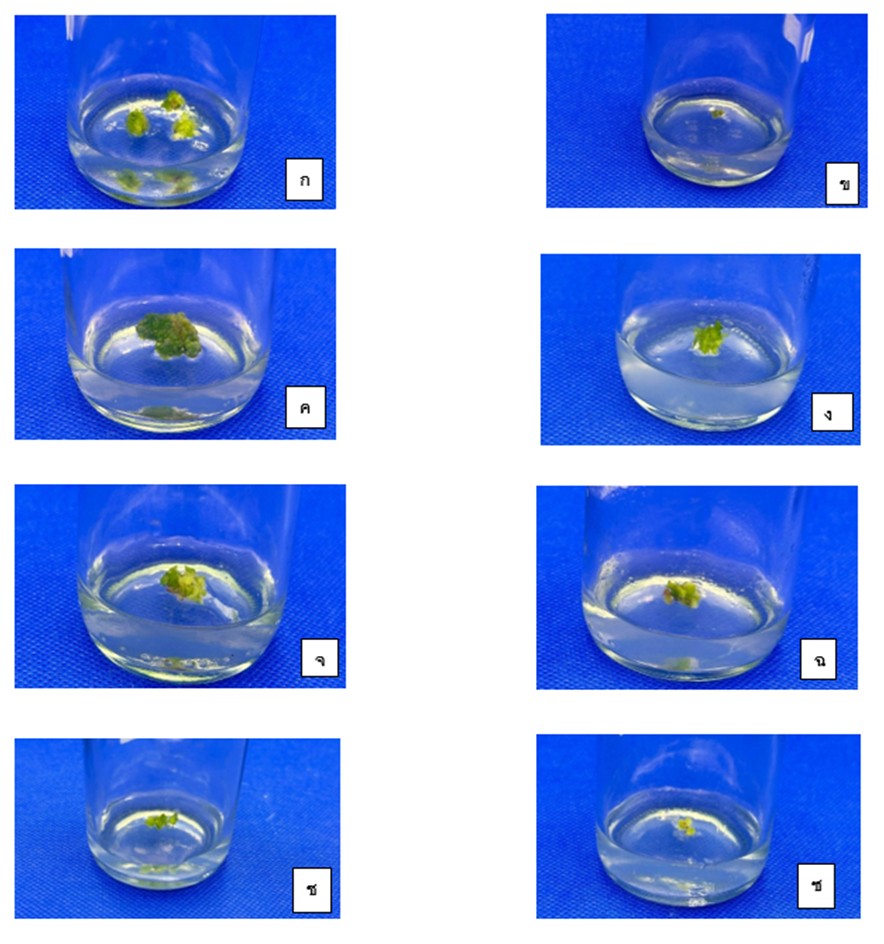
The toothbrush orchid is a monocotyledonous plant. It is classified in the Dendrobium genus, which is an important and outstanding economic flowering plant. It is an orchid that is sold both domestically and internationally due to its overall toothbrush-like design, both in the flower arrangement and stem. Recently, there are fewer toothbrush orchids now. Therefore, the objective of this study was to study the effects of Ethylmethane sulfonate (EMS) and detection of genetic variation in toothbrush orchids using simple sequence repeat (SSR) marker. EMS solution was used to soak a 0.5 cm piece of the protocorm like bodies (PLBs) at concentrations of 0.5, 1.0, and 2.0%. Thereafter, they were cultured on VW medium supplemented with 1 mg/l BA, 20 g/l sucrose, adjusted to pH 5.7 and solidified with 0.2 % phytagel. The cultures were maintained at 26 ±2°C under light at intensity of 3,000 lux for 14 hours per day. After culturing for 30 days, the results showed that PLBs immersed in EMS solution at 1.7% for 90 minutes gave the highest average survival rates at 50% (LD50). For the development of PBLs, PBLs were derived with immersed in 0.5 % EMS for 60 minutes gave the highest PLBs induction (7.37%) and average number of PLBs (1.41 PLBs/explant) after culturing for 8 weeks. For detection of genetic variation, a total of 9 SSR primers were used, including EgCIR0409, EgCIR0905, EgCIR0781, EgCIR0446, EgCIR1772, EgCIR0337, EgCIR0337, EgCIR0243, mEgCIR0465, and mEgCIR008. The results found that only one primer gave polymorphic banding. EgCIR0905 primer gave polymorphic banding at 50% and specific DNA banding size at 200 bp.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Orchidtropical. Available online: https://www.orchidtropical.com/. (access on 7 October 2022).
Agatha, S.; Fenny, I.; Sulistyo, E.; Popy, H. Induction of protocorm-like bodies (PLBs) Phalaenopsis spp. hybrids mutation through ultraviolet irradiation (UV254) and ethyl methane sulfonate (EMS). Journal of Applied Agricultural Sciences. 2023, 7(1), 1-15. DOI: 10.25047/agriprima.v7i1.512. https://doi.org/10.25047/agriprima.v7i1.512
Talebi, A.B.; Talebi, A.B.; Shahrokhifar, B. Ethyl methane sulphonate (EMS) induced mutagenesis in Malaysian rice (cv.MR219) for lethal dose determination. American Journal of Plant Sciences. 2012, 3. 1661-1665. http://dx.doi.org/10.4236/ajps.2012.312202.
Li, C.; Dong, N.; Zhao, Y.; Wu, S.; Liu, Z.; Zhai, J. A review for the breeding of orchids: Current achievements and prospects. Horticultural Plant Journal. 2021, 7(5), 380-392. https://doi.org/10.1016 /j.hpj.2021.02.006 https://doi.org/10.1016/j.hpj.2021.02.006
Romiyadi, R.; Komariah, A.; Amien, S. Keragaan tiga jenis planletanggrek Phalaenopsis asal Protocorm yang diinduksi Ethyl Methyl Sulfonate (EMS) secara in vitro. Kultivasi. 2018, 17(1), 596-607. https://doi.org /10.24198/kultivasi.v17i1.16077 https://doi.org/10.24198/kultivasi.v17i1.16077
Qosim, W. A.; Istifadah, N.; Djatnika, I.; Y. Pengaruh. Mutagen Etil Metan Sulfonat terhadap Kapasitas Regenerasi Tunas Hibrida Phalaenopsis, in vitro. Jurnal Hortikultura. 2016, 22(4), 360-365. https://doi.org /10.21082/jhort.v22n4.2012. https://doi.org/10.21082/jhort.v22n4.2012.p360-365
Avivi, S.; Hariyono, K.; Hartatik, S.; Pertanian, F.; Jember, U. Analisis Pendugaan Parameter Genetik pada Genotipe Tebu Mutan. Agriprima: Journal of Applied Agricultural Sciences, 2022, 26(2), 124-134. https://agriprima.polije.ac.id/index.php/journal/article/view/v6i2-c https://doi.org/10.25047/agriprima.v6i2.456
Arisha, M, H.; Shah, S.N.M.; Gong, Z. Ethyl methane sulfonate induced mutations in M2 gene ratio and physiological variations in M1 generation of peppers (Capsicum annum L.). Fronteirs in Plant Science, 2025, 6(399), 1-7. doi:10.3389/fpls.2015.00399. https://doi.org/10.3389/fpls.2015.00399
Tadmor, Y.; Katzir, N.; Meir, A. Induced mutagenesis to augment the natural genetic variability of melon (Cucumismelo L.). Israel Journal of Plant Sciences, 2007, 55(2), 159-169. doi: 10.1560/IJPS.55.2.159 https://doi.org/10.1560/IJPS.55.2.159
Temnykh, S.; Park, W.D.; Ayers, N. Mapping and genome organization of microsatellite sequence in rice (Oryza sativa L.). Theoretical and Applied Genetics, 2000, 100(5), 697-712. doi: 0.1007/s001220051342. https://doi.org/10.1007/s001220051342
Prasanna, B.M; Pixley, K.; Warburton, M.L.; Xie, C.X. Molecular marker-assisted breeding options for maize improvement in Asia. Molecular Breeding, 2010, 26(2), 339-356. doi:10.1007/2Fs11032-009-9387-3. https://doi.org/10.1007/s11032-009-9387-3
Ciampi, A.Y.; Azevedo, V.C.R.; Gaiotto, F.A. Isolation and characterization of microsatellite loci for Hymenaea courbaril and transferability to Hymenaea stigonocarpa, two tropical timber species. Molecular Ecology Resources, 2008, 8(5), 1074-1077. doi: 10.1111/j.1755-0998.2008.02159.x https://doi.org/10.1111/j.1755-0998.2008.02159.x
Lorieux, M.; Ndjiondjop, M.N.; Ghesquiere, A. A first interespecific Oryza sativa x Oryza glaberrima microsatellite based genetic linkage map. Theoretical and Applied Genetics, 2000, 100(3-4), 593-601. doi: 10.1007/2Fs001229900061. https://doi.org/10.1007/s001220050078
Ramasoot, S.; Weerapong, M.; Keawsaard, Y.; Ritchuay, S.; Rotduang, P. Enhance efficiency propagation and conservation of toothbrush orchid in vitro. Songklanakarin Journal of Plant Science, 2022, 9(1), 15-23.
Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus. 1990, pp.12- 15. https://doi.org/10.2307/2419362
Thawaro, S.; Te-chato, S. Application of molecular markers in the hybrid verification and assessment of somaclonal variation from oil palm propagated in vitro. Science Asia, 2009, 35, 142-149. http://dx.doi.org/ 10.2306/scienceasia1513-1874.2009.35.142 https://doi.org/10.2306/scienceasia1513-1874.2009.35.142
Sirinya, M.; Te-chato, S. Use of ems to induce mutation in Dendrobium friedericksianum Rchb.f., Journal of Agriculture, 2008, 24(2), 153-164.
Samala, S.; Te-chato, S.; Yenchon, S. Effect of ethyl methanesulphonate (EMS) on Dendrobium Sonia. Khon kaen agr. Journal, 2014, 42(3), 506-511.
Arisha, M. H.; Liang, B.K.; Muhammad, S. S. N.; Gong, Z.H.; Li, D.W. Kill curve analysis and response of first-generation Capsicum annuum L. B12 cultivar to ethyl methane sulfonate. Genetics and Molecular Research, 2014, 13(4), 10049-10061. https://doi.org/10.4238/2014.November.28.9 https://doi.org/10.4238/2014.November.28.9
Romeida, A.; Stujahjo, S. H.; Purwito, A.; Sukma, D.; Rurstikawati, S. Induksi Mutasi Protocorm Like Bodies (PLBs) Anggrek Spathoglottis plicataBlume. Aksesi Bengkulu pada Sebelas Taraf Dosis Iradiasi Sinar Gamma. Prosiding Simposium Dan Seminar Bersama PERAGI-PERHORTI-PERIPIHIGI Mendukung Kedaulatan Pangan Dan Energi Yang Berkelanjutan, 2012, 381-387. https://repository.ipb.ac.id/handle /123456789/59898
Sharmin, A.; Hoque, M.E.; Haque, M.M.; Khatun, F. Molecular diversity analysis of some chilli (Capsicum spp.) genotypes using SSR markers. American Journal of Plant Science, 2018, 9(3), 368-379. doi: 10.4236/ ajps.2018.93029. https://doi.org/10.4236/ajps.2018.93029
Botstein, D.; White, R.L.; Skolnik, M.; Davis, R.W. Construc-tion of a genetic linkage map in man using restriction fragment lenght polymorphisms. American Journal of Human Genetics, 1980, 32(3), 314-331.
Greene, E.A.; Codomo, C.A.; Taylor, N.E. Spectrum of chemically induced mutations from a large scale reverse genetic screen in Arabidopsis. Genetics, 2003, 164(2), 731-740. https://doi.org/10.1093/genetics/164.2.731
Powell, W.; Morgante, M.; Andre, C. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Molecular Breeding, 1996, 2(3), 225-238. doi: 10.1007/BF00564200. https://doi.org/10.1007/BF00564200
Zhang, G.; Wang, Y.; Guo, Y. Characterization and mapping of QTLs on chromosome 2D for grain size and yield traits using a mutant line induced by EMS in wheat. The Crop Journal, 2015, 3(2), 135-144. doi: 10.1016/j.cj.2014.11.002 https://doi.org/10.1016/j.cj.2014.11.002
Tautz, D.; Renz, M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Research, 1987, 12(10), 4127-4138. doi: 10.1093/nar/12.10.4127. https://doi.org/10.1093/nar/12.10.4127
Kim, Y.S.; Schumaker, K.S.; Zhu, J.K. EMS Mutagenesis of Arabidopsis. In: Salinas J, Sanchez-Serrano JJ (eds) Arabidop-sis Protocols. Methods in Molecular Biology; New Jersey, Hu-mana Press. 2006. pp 101-103. doi: 10.1385/1-59745-003-0:101. https://doi.org/10.1385/1-59745-003-0:101
Boonsrangsom, T.; Pongtongkam, P.; Masuthon, S.; Peyachoknagu, S. Development of microsatellite markers for Dendrobium orchids. Thai Journal of Genetics, 2008, 1(1), 47-56.