Assessing the Antioxidant Activity and Anti-inflammatory Potency on Lipopolysaccharide-induced RAW 264.7 Macrophages of Eleven Methanolic Extracts Indigenous Vegetables
Main Article Content
Abstract
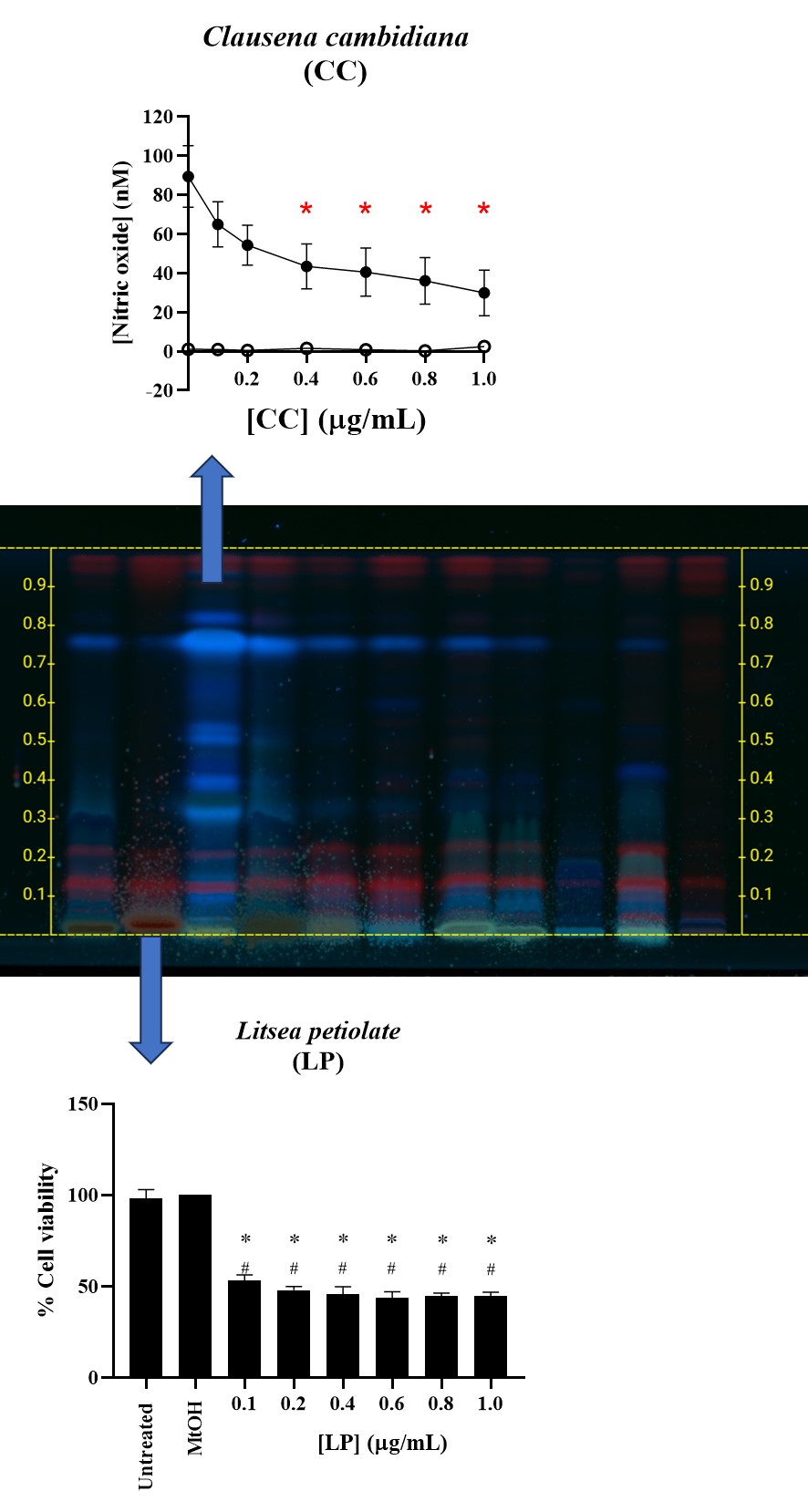
Indigenous vegetables have long been part of the traditional diet in southern Thailand, often paired with spicy foods. This study aimed to explore the total phenolic and flavonoid contents of eleven commonly consumed indigenous vegetables in Nakhon Si Thammarat province, while also evaluating their antioxidant and anti-inflammatory properties through methanolic crude extracts. The findings revealed that Litsea petiolate (LP) contained the highest amount of flavonoids, with 270.90 ± 6.175 mg QE/g extract, whereas Anacardium occidentale (AO) showed the greatest phenolic content at 160.40 ± 1.32 mg GAE/g extract. In terms of antioxidant properties, Glochidion wallichianum (GW) demonstrated significant activity through the FRAP assay, yielding 484.08 ± 10.010 μmole TE/g extract and 4,115.36 ± 100.00 μmole Fe²⁺/g extract. Meanwhile, AO exhibited the most substantial results in the DPPH assay, with an 85.99 ± 0.762 mg/ml capacity. The IC50 values of AO (7.39 ± 0.176 mg/ml) and GW (7.70 ± 0.820 mg/ml) surpassed that of ascorbic acid, indicating their high antioxidant potential. In terms of anti-inflammatory effects, Crassocephalum crepidioides (CC) effectively inhibited nitric oxide production at different concentrations, while the LP extract impacted the viability of the RAW264.7 cell line, a distinction not observed in the other vegetables studied. These results underscore the significant bioactive potential of these indigenous vegetables. This not only enhances their value in sustainable food systems but also highlights their educational importance in promoting knowledge of the plant cycle for future generations
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
El-Saber, B.G.; Magdy, B.A., El-Mleeh, A., Abdel-Daim, M.M.; Prasad, D.H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10(3), 352. https://doi.org/10.3390/biom10030352
Carlsen, M.H.; Halvorsen, B.L.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; Barikmo, I.; Berhe, N.; Willett, W.C.; Phillips, K.M.; Jacobs, J.D.R..; Blomhoff, R. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. J Nutr 2010, 9, 3. https://doi.org/10.1186/1475-2891-9-3.
Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; Alam, M.; Mohibbullah, M.; Haque, M.N.; Jahan, I.; Hossain, M.T.; Afrin, T.; Rahman, M.M.; Tahjib-Ul-Arif, M.; Mitra, S.; Oktaviani, D.F.; Khan, M.K.; Choi, H.J.; Moon, I.S.; Kim, B. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021, 13(6), 1784. https://doi.org/10.3390/nu13061784.
Fazmiya, M.J.A.; Sultana, A.; Rahman, K.; Heyat, M.B.B.; Akhtar, F.; Khan, S.; Appiah, S.C.Y. Current Insights on Bioactive Molecules, Antioxidant, Anti-Inflammatory, and Other Pharmacological Activities of Cinnamomum camphora Linn. Oxid Med Cell Longev 2022, 2022, 9354555. https://doi.org/10.1155/2022/9354555
Abbas, M.W.; Hussain, M.; Qamar, M.; Ali, S.; Shafiq, Z.; Wilairatana, P.; Mubarak, M.S. Antioxidant and Anti-Inflammatory Effects of Peganum harmala Extracts: An In Vitro and In Vivo Study. Molecules 2021, 26(19), 6084. https://doi.org/10.3390/molecules26196084
Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch. Toxicol. 2016, 90(8), 1817-40. https://doi.org/10.1007/s00204-016-1744-5.
Ożarowski, M.; Karpiński, T.M. Extracts and Flavonoids of Passiflora Species as Promising Anti-inflammatory and Antioxidant Substances. Curr. Pharm. Des. 2021, 27(22), 2582-2604. https://doi.org/10.2174/1381612826666200526150113.
Higuchi, H.; Granger, D.N.; Saito, H.; Kurose, I. Assay of antioxidant and anti-inflammatory activity of nitric oxide in vivo. Methods Enzymol 1999, 301, 424-436. https://doi.org/10.1016/S0076-6879(99)01106-4.
Monforte, M.T.; Smeriglio, A.; Germanò, M.P.; Pergolizzi, S.; Circosta, C.; Galati, E.M. Evaluation of antioxidant, antiinflammatory, and gastroprotective properties of Rubus fruticosus L. fruit juice. Phytother Res 2018, 32(7), 1404-1414. https://doi.org/10.1002/ptr.6078.
Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9(12), 1309. https://doi.org/10.3390/antiox9121309.
Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci 2021, 22(7), 3380. https://doi.org/10.3390/ijms22073380.
Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of Anti-Inflammatory Herbal Medicines. Adv Pharmacol Sci 2016, 2016, 9130979. https://doi.org/10.1155/2016/9130979.
Franchimont, P. Clinical and biochemical assessment of anti-inflammatory substances. Agents Actions 1976, 6(1-3), 369-72. https://doi.org/10.1007/BF01972257.
Popescu, I.D.; Codrici, E.; Mihai, S.; Luntraru, C.M.; Neagu, M.; Tanase, C. In vitro assessment of the cytotoxicity and anti-inflammatory properties of a novel dietary supplement. Exp Ther Med 2021, 22(4), 1170. https://doi.org/10.3892/etm.2021.10604.
Jannus, F.; Medina-O'Donnell, M.; Neubrand, V.E.; Marín, M.; Saez-Lara, M.J.; Sepulveda, M.R.; Rufino-Palomares, E.E.; Martinez, A.; Lupiañez, J.A.; Parra, A.; Rivas, F.; Reyes-Zurita, F.J. Efficient In Vitro and In Vivo Anti-Inflammatory Activity of a Diamine-PEGylated Oleanolic Acid Derivative. Int J Mol Sci 2021, 22(15), 8158. https://doi.org/10.3390/ijms22158158.
Office of the Royal Development Projects Board. Summary of Royal Development Project in Nakhon Si Thammarat. Retrieved from http://www.rdpb.go.th/rdpb/projectData/files/south/summary_roy_southProject62.pdf, 2020.
United Nation Economic and Social Commivision for Asia and the Pacific. Nakhon Si Thammarat, Thailand. pp.14. Retrieved from https://www.unescap.org/. 2020.
Damsri, U. Indigenous vegetables in Changwat Nakhon Si Thammarat. Bangkok, Thailand: Chulalongkorn University, Ms. Thesis. ISBN 974-582-110-1. 1992, 353.
Sintupachee, S.; Rattanachoo, P.; Promproa, S. Alpha-Mangostin Quality and Quantity Analysis in Nakhon Si Thammarat Mangosteen Pericarp Using Thin-Layer Chromatography. Malaysian Journal of Sustainable Agricultures 2022, 6(1), 51-56.
Silver J. Let Us Teach Proper Thin Layer Chromatography Technique. J. Chem. Educ., 2020, 97(12), 4217-4219. https://doi.org/10.1021/acs.jchemed.0c00437
Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem 2017, 240, 212-221. https://doi.org/10.1016/j.foodchem.2017.07.095.
Fattahi, S.; Zabihi, E.; Abedian, Z.; Pourbagher, R.; Motevalizadeh A.A.; Mostafazadeh. A.; Akhavan-Niaki, H. Total Phenolic and Flavonoid Contents of Aqueous Extract of Stinging Nettle and In Vitro Antiproliferative Effect on Hela and BT-474 Cell Lines. Int J Mol Cell Med, 2014, 3(2), 102-107. PMID: 25035860; PMCID: PMC4082812.
Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal. Biochem 1996, 239(1), 70-6. https://doi.org/10.1006/abio.1996.0292.
Promden, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Structure and antioxidant activity relationships of isoflavonoids from Dalbergia parviflora. Molecules 2014, 19, 22-26. https://doi.org/10.3390/molecules19022226.
Vishwakarma, A.; Wany, A.; Pandey, S.; Bulle, M.; Kumari, A.; Kishorekumar, R.; Igamberdiev, A.U.; Mur, L.A.J.; Gupta, K.J. Current approaches to measure nitric oxide in plants. J Exp Bot 2019, 70(17), 4333–4343. https://doi.org/10.1093/jxb/erz242
Promden, W.; Chanvorachote, P.; Viriyabancha, W.; Sintupachee, S.; De-Eknamkul, W.Maclura cochinchinensis (Lour.) Corner Heartwood Extracts Containing Resveratrol and Oxyresveratrol Inhibit Melanogenesis in B16F10 Melanoma Cells. Molecules 2014, 29(11), 2473. https://doi.org/10.3390/molecules29112473.
Baptista, A.B.; Sarandy, M.M.; Gonçalves, R.V.; Novaes, R.D.; Gonçalves da Costa, C.; Leite, J.P.V.; Peluzio, M.D.C.G. Antioxidant and Anti-Inflammatory Effects of Anacardium occidentale L. and Anacardium microcarpum D. Extracts on the Liver of IL-10 Knockout Mice. J Evid Based Complementary Altern Med 2020, 2020, 3054521. https://doi.org/10.1155/2020/3054521.
Sassi, A.; Normah, H.; Khattak, M.M.A.K.; Hanapi, M.J. Analysis of phenolic profile, total phenolic content and antioxidant activity in Anacardium occidentale leaves. Food Research 2022, 6(1), 20-26. https://doi.org/10.26656/fr.2017.
Amira, P.O.; Daramola, A.S.; Muoghalu, C.E.; Ojo, O.B. (2020). Comparative Studies on Phytochemical Screening and in Vitro Antioxidant Activities of Aqueous Extracts of Anacardium Occidentale Leaves and Nuts. Eur J Biol Biotechnol 2020, 1(4). https://doi.org/10.24018/ejbio.2020.1.4.49.
Ambarwati, N.S.S.; Elya, B.; Mahayasih, P.G.M.W.; Awang, M.S.N.; Omar, H. Antioxidant activity of Litsea petiolata Hk. F. J. Phys.: Conf. Ser. 1869, 012055. https://doi.org/10.1088/1742-6596/1869/1/012055
Wongnen, C.; Ruzzama, N.; Chaijan, M.; Cheong, L.Z.; Panpipat, W. Glochidion wallichianum Leaf Extract as a Natural Antioxidant in Sausage Model System. Foods 2022, 11(11), 1547. https://doi.org/10.3390/foods11111547.
Samuhasaneeto, S.; Punsawad, C.; Chaniad, P. Protective Effects of Glochidion wallichianum Mull. Arg. on Ethanol-induced Liver Injury in Rats. Trends Sci 2022, 19(12), 4609. https://doi.org/10.48048/tis.2022.4609.
Can, N.M.; Thao, D.T.P. Wound Healing Activity of Crassocephalum crepidioides (Benth.) S. Moore. Leaf Hydroethanolic Extract. Oxid Med Cell Longev 2022, 2020, 2483187. https://doi.org/10.1155/2020/2483187.
Wijaya, S.; Kang, T.; Teng, K.; Mustafa, W.; Wiart, C. Antioxidant, Anti-Inflammatory, Cytotoxicity and Cytoprotection Activities of Crassocephalum Crepidioides (Benth.) S. Moore. Extracts and Its Phytochemical Composition. Eur. J. Sci. Res 2011, 67(1). 1-10. https://doi.org/10.4061/2011/768673.
Manik, M.K.; Islam, S.M.A.; Wahid, M.dA.; Morshed, M.M.; Kamal, S.; Islam, Md.S.; Ahmed, Kh.T. Investigation of In Vitro Antioxidant, Antimicrobial and Thrombolytic Activity of the Exocarp of Spondias pinnata (Anacardiaceae). Can Chem Trans 2013, 3(1), 191-201. https://doi.org/10.13179/canchemtrans.2013.01.03.0029.
Bahramikia, S.; Yazdanparast, R. Antioxidant efficacy of Nasturtium officinale extracts using various in vitro assay systems. J Acupunct Meridian Stud 2010, 3(4), 283-290. https://doi.org/10.1016/S2005-2901(10)60049-0.
Klimek-Szczykutowicz, M.; Dziurka, M.; Blažević, I.; Đulović, A.; Miazga-Karska, M.; Klimek, K.; Ekiert, H.; Szopa, A. Precursor-Boosted Production of Metabolites in Nasturtium officinale Microshoots Grown in Plantform Bioreactors, and Antioxidant and Antimicrobial Activities of Biomass Extracts. Molecules 2021, 26(15), 4660. https://doi.org/10.3390/molecules26154660.
Antoniak, K.; Studzińska-Sroka, E.; Szymański, M.; Dudek-Makuch, M.; Cielecka-Piontek, J.; Korybalska, K. Antiangiogenic, Anti-Inflammatory and Antioxidant Properties of Bidens tripartite Herb, Galium verum Herb and Rumex hydrolapathum Root. Molecules 2023, 28(13), 4966. https://doi.org/10.3390/molecules28134966.
Zhang, S.; Huang, Y.; Li, Y.; Wang, Y.; He, X. Anti-neuroinflammatory and antioxidant phenylpropanoids from Chinese olive. Food chem 2019, 15;286:421-427. https://doi.org/10.1016/j.foodchem.2019.02.031.
de Abreu Costa, L.; Henrique Fernandes Ottoni, M.; Dos Santos, M.G.; Meireles, A.B.; Gomes de Almeida, V.; de Fátima Pereira, W.; Alves de Avelar-Freitas, B.; Eustáquio Alvim Brito-Melo, G. Dimethyl Sulfoxide (DMSO) Decreases Cell Proliferation and TNF-α, IFN-γ, and IL-2 Cytokines Production in Cultures of Peripheral Blood Lymphocytes. Molecules 2017, 22(11), 1789. https://doi.org/10.3390/molecules22111789. PMID: 29125561; PMCID: PMC6150313.