Methane Oxidation Rates and Efficiencies Across Four Distinct Soil Environments: Implications for Greenhouse Gas Mitigation
Main Article Content
Abstract
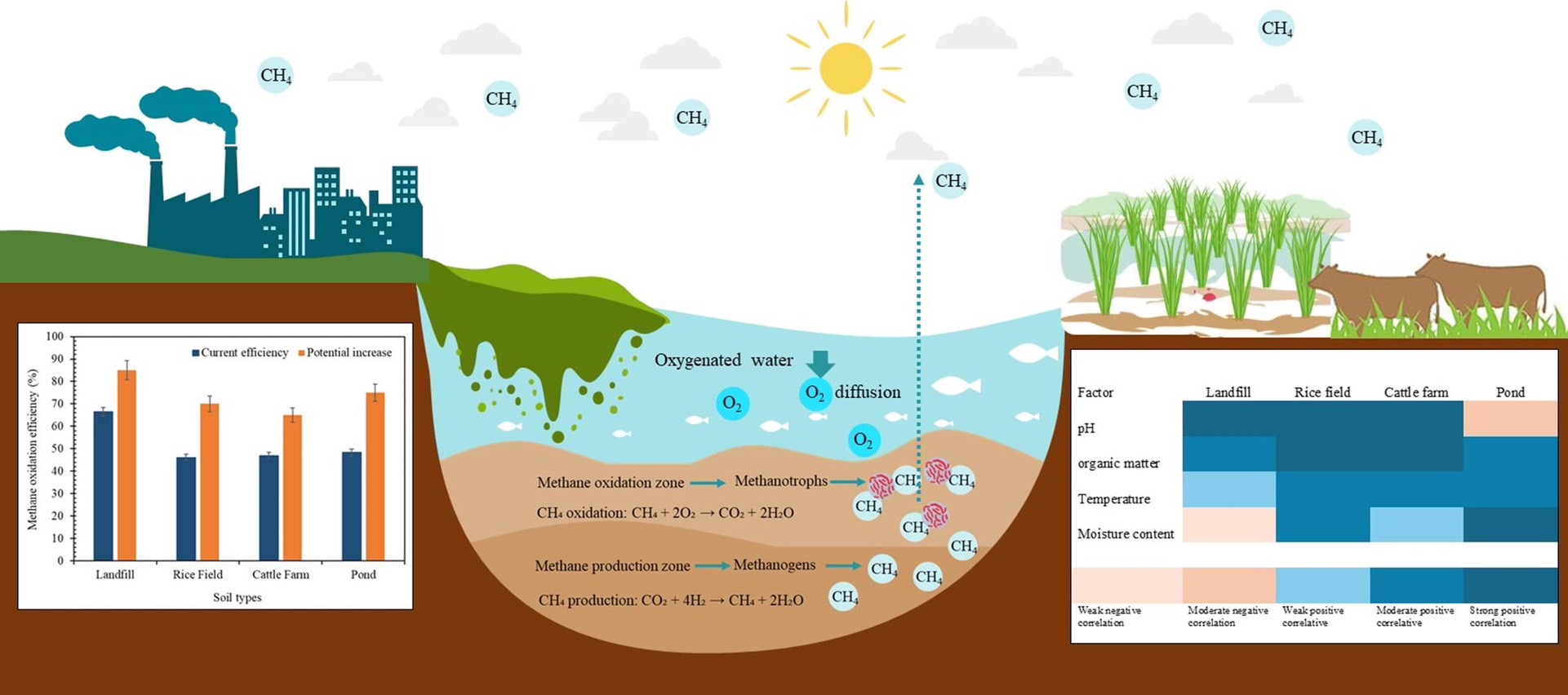
Methane oxidation by soil microorganisms is crucial in mitigating greenhouse gas emissions. This study investigated methane oxidation potential across four distinct soil environments through standardized laboratory enrichment cultures. Soil samples were collected from landfill-cover soils, rice fields, cattle farms, and pond sediments, with environmental parameters monitored to understand their influence on oxidation rates and efficiencies. Using gas chromatography analysis, we quantified methane oxidation under controlled conditions. Statistical analysis revealed significant differences in oxidation rates across soil types. Landfill cover soils exhibited the highest oxidation rate of 0.39 μmol-CH₄/g-soil dry weight/h and efficiency of 66.5 %. Pond sediments, cattle farm soils, and rice field soils followed with rates of 0.29, 0.28, and 0.27 μmol-CH₄/g-soil dry weight/h, respectively. Oxidation efficiencies for these environments ranged from 46.1% to 48.4%. pH and organic matter content showed strong positive correlations with oxidation rates across all soil types, while environmental moisture content effects varied. The superior performance of landfill soils was attributed to optimal environmental conditions and stable substrate availability. This analysis revealed significant potential for enhancing oxidation efficiencies: landfill soils from 66.5% to 75-85%, rice fields from 46.1% to 60-70%, cattle farms from 47.0% to 55-65%, and pond sediments from 48.4% to 60-75%. Implementing optimized management strategies could reduce methane emissions by 70-90% in landfills, 30-50% in agricultural systems, and 40-60% in aquatic environments compared to current practices. This study highlights the substantial potential for enhancing biological methane oxidation across diverse ecosystems and emphasizes the need for targeted management approaches to optimize methane mitigation strategies.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2021, pp. 2391. https://doi.org/10.1017/9781009157896.
World Meteorological Organization, 2022. State of Climate in 2021: Extreme events and major impacts. Available online: https://wmo.int/news/media-centre/state-of-climate-2021-extreme-events-and-major-impacts. (accessed on 7 September 2024).
Etminan, M.; Myhre, G.; Highwood, E.J.; Shine, K.P. Radiative forcing of carbon dioxide, methane, and nitrous oxide: A significant revision of the methane radiative forcing. Geophysical Research Letters 2016, 43(24), 12614-12623. https://doi.org/10.1002/2016GL071930.
Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiological Reviews 1996, 60(2), 439-471. https://doi.org/10.1128/mr.60.2.439-471.1996.
Wallenius, A.J.; Martins, P.D.; Slomp, C.P.M. Jetten, M.S. Anthropogenic and environmental constraints on the microbial methane cycle in coastal sediments. Frontiers in Microbiology 2021, 12. https://doi.org/10.3389/fmicb.2021.631621.
Knief, C. Diversity and habitat preferences of cultivated and uncultivated aerobic methanotrophic bacteria evaluated based on pmoA as molecular marker. Frontiers in Microbiology 2015, 6, 170928. https://doi.org/10.3389/fmicb.2015.01346.
Liu, Y.; Ding, C.; Xu, X.; Wang, K.; Li, Y.; Pan, H.; Zhang, Q.; Dumont, M.G.; Di, H.; Xu, J.; Li, Y. Atmospheric methane oxidation is affected by grassland type and grazing and negatively correlated to total soil respiration in arid and semiarid grasslands in Inner Mongolia. Soil Biology and Biochemistry 2022, 173, 108787. https://doi.org/10.1016/j.soilbio.2022.108787.
Fenibo, E.O.; Selvarajan, R.; Wang, H.; Wang, Y.; Abia, A.L.K. Untapped talents: Insight into the ecological significance of methanotrophs and its prospects. Science of The Total Environment 2023, 903, 166145. https://doi.org/10.1016/j.scitotenv.2023.166145.
Rahalkar, M.C.; Khatri, K.; Pandit, P.; Bahulikar, R.A.; Mohite, J.A. Cultivation of important methanotrophs from Indian rice fields. Frontiers in Microbiology 2021, 12. https://doi.org/10.3389/fmicb.2021.669244.
Wang, J.; Xia, F.; Bai, Y.; Fang, C.; Shen, D.; He, R. Methane oxidation in landfill waste biocover soil: Kinetics and sensitivity to ambient conditions. Waste Management 2011, 31(5), 864-870. https://doi.org/ 10.1016/j.wasman.2011.01.026.
Zhou, H.; Tao, F.; Chen, Y.; Yin, L.; Li, Y.; Wang, Y.; Su, C. Paddy rice methane emissions, controlling factors, and mitigation potentials across Monsoon Asia. Science of The Total Environment 2024, 935, 173441. https://doi.org/10.1016/j.scitotenv.2024.173441.
Dutaur, L.; Verchot, L.V.A global inventory of the soil CH4 sink, Global Biogeochem. Cycles 2007, 21, GB4013. https://doi.org/10.1029/2006GB002734.
Hansen, L.V.; Brændholt, A.; Tariq, A.; Jensen, L.S.; Peixoto, L.E.; Petersen, S.O.; Bruun, S. Methane uptake rates across different soil types and agricultural management practices in Denmark. Agriculture, Ecosystems & Environment 2024, 363, 108878. https://doi.org/10.1016/j.agee.2023.108878.
Bastviken, D.; Cole, J.J.; Pace, M.L.; Van de Bogert M.C. Fates of methane from different lake habitats: Connecting whole-lake budgets and CH4 emissions. Journal of Geophysical Research 2008, 113, G02024. https://doi.org/10.1029/2007JG000608.
Grasset, C.; Mendonça, R.; Villamor Saucedo, G.; Bastviken, D.; Roland, F.; Sobek, S. Large but variable methane production in anoxic freshwater sediment upon addition of allochthonous and autochthonous organic matter. Limnology and Oceanography 2018, 63(4), 1488-1501. https://doi.org/10.1002/lno.10786.
Whittenbury, R.; Phillips, K.C.; Wilkinson, J.F. Enrichment, isolation and some properties of methane-utilizing bacteria. Journal of General Microbiology 1970, 61(2), 205-218. https://doi.org/10.1099/00221287-61-2-205.
Vishniac, W.; Santer, M. The Thiobacilli. Bacteriological Reviews 1957, 21(3), 195-213. https://doi.org/ 10.1128/br.21.3.195-213.1957.
Scheutz, C.; Kjeldsen, P.; Bogner, J.E.; De Visscher, A.; Gebert, J.; Hilger, H.A.; Huber-Humer, M.; Spokas, K. Microbial methane oxidation processes and technologies for mitigation of landfill gas emissions. Waste Management & Research 2009, 27(5), 409-55. https://doi.org/10.1177/0734242X09339325.
Chanton, J.; Abichou, T.; Langford, C.; Spokas, K.; Hater, G.; Green, R.; Goldsmith, D.; Barlaz, M.A. Observations on the methane oxidation capacity of landfill soils. Waste Management 2011, 31(5), 914-925. https://doi.org/10.1016/j.wasman.2010.08.028.
Huber-Humer, M.; Gebert, J.; Hilger, H. Biotic systems to mitigate landfill methane emissions. Waste Management & Research 2008, 26(1), 33-46. https://doi.org/10.1177/0734242X07087977.
Bodelier, P.L.; Roslev, P.; Henckel, T.; Frenzel, P. Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 2000, 403(6768), 421-424. https://doi.org/10.1038/35000193.
Krüger, M.; Frenzel, P.; Conrad, R. Microbial processes influencing methane emission from rice fields. Global Change Biology 2002, 7(1), 49-63. https://doi.org/10.1046/j.1365-2486.2001.00395.x.
Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. European Journal of Soil Biology 2001, 37(1), 25-50. https://doi.org/10.1016/S1164-5563(01)01067-6.
Yao, X.; Wang, J.; Hu, B. How methanotrophs respond to pH: A review of ecophysiology. Frontiers in Microbiology 2022, 13. https://doi.org/10.3389/fmicb.2022.1034164.
Neue, H.; Wassmann, R.; Kludze, H.; Bujun, W.; Lantin, R.S. Factors and processes controlling methane emissions from rice fields. Nutrient Cycling in Agroecosystems 1997, 49, 111–117. https://doi.org/10.1023/ A:1009714526204.
Han, Y.; Qi, Z.; Chen, P.; Zhang, Z.; Zhou, X.; Li, T.; Du, S.; Xue, L. Water-saving irrigation mitigates methane emissions from paddy fields: The role of iron. Agricultural Water Management 2024, 298, 108839. https://doi.org/10.1016/j.agwat.2024.108839.
Tang, S.; Ma, L.; Wei, X.; Tian, D.; Wang, B.; Li, Z.; Zhang, Y.; Shao, X. Methane emissions in grazing systems in grassland regions of China: A synthesis. Science of The Total Environment 2019, 654, 662-670. https://doi.org/10.1016/j.scitotenv.2018.11.102.
Seghers, D.; Top, E.M.; Reheul, D.; Bulcke, R.; Boeckx, P.; Verstraete, W.; Siciliano, S.D. Long-term effects of mineral versus organic fertilizers on activity and structure of the methanotrophic community in agricultural soils. Environmental Microbiology 2003, 5(10), 867-77. https://doi.org/10.1046/j.1462-2920.2003.00477.x.
Zhang, W.; Sheng, R.; Zhang, M.; Xiong, G.; Hou, H.; Li, S.; Wei, W. Effects of continuous manure application on methanogenic and methanotrophic communities and methane production potentials in rice paddy soil. Agriculture, Ecosystems & Environment 2018, 258, 121-128. https://doi.org/10.1016/j.agee.2018.02.018.
Yang, Y.; Chen, J.; Tong, T.; Li, B.; He, T.; Liu, Y.; Xie, S. Eutrophication influences methanotrophic activity, abundance and community structure in freshwater lakes. Science of The Total Environment 2019, 662, 863-872. https://doi.org/10.1016/j.scitotenv.2019.01.307.
Norði, K.Á.; Thamdrup, B. Nitrate-dependent anaerobic methane oxidation in a freshwater sediment. Geochimica et Cosmochimica Acta 2014, 132, 141-150. https://doi.org/10.1016/j.gca.2014.01.032.
Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Global Change Biology 2013, 19(5), 1325-46. https://doi.org/10.1111/gcb.12131.
Ulumuddin, Y.I.; Sugoro, I.; Beavis, S.; Roderick, M.; Eggins, S.; Muarif, M.R. Characterisation of methane production pathways in sediment of overwashed mangrove forests. Forests 2023, 14(3), 564. https://doi.org/10.3390/f14030564.
Dedysh, S.N. Exploring methanotroph diversity in acidic northern wetlands: Molecular and cultivation-based studies. Microbiology 2009, 78, 655–669. https://doi.org/10.1134/S0026261709060010.
Borrel, G.; Jézéquel, D.; Biderre-Petit, C.; Morel-Desrosiers, N.; Morel, J.; Peyret, P.; Fonty, G.; Lehours, A. Production and consumption of methane in freshwater lake ecosystems. Research in Microbiology 2011, 162(9), 832-847. https://doi.org/10.1016/j.resmic.2011.06.004.
Cabrol, L.; Thalasso, F.; Gandois, L.; Sepulveda-Jauregui, A.; Martinez-Cruz, K.; Teisserenc, R.; Tananaev, N.; Tveit, A.; Svenning, M. M.; Barret, M. Anaerobic oxidation of methane and associated microbiome in anoxic water of Northwestern Siberian lakes. Science of The Total Environment 2020, 736, 139588. https://doi.org/10.1016/j.scitotenv.2020.139588.
He, R.; Wooller, M. J.; Pohlman, J. W.; Quensen, J.; Tiedje, J. M.; Leigh, M. B. Shifts in identity and activity of methanotrophs in arctic lake sediments in response to temperature changes. Applied and Environmental Microbiology 2012, 78(13), 4715-4723. https://doi.org/10.1128/AEM.00853-12.
Yan, X.; Akiyama, H.; Yagi, K.; Akimoto, H. Global estimations of the inventory and mitigation potential of methane emissions from rice cultivation conducted using the 2006 Intergovernmental Panel on Climate Change Guidelines. Global Biogeochemical Cycles 2009, 23(2). https://doi.org/10.1029/2008GB003299.
Sepulveda-Jauregui, A.; Hoyos-Santillan, J.; Martinez-Cruz, K.; Walter Anthony, K.M.; Casper, P.; Belmonte-Izquierdo, Y.; Thalasso, F. Eutrophication exacerbates the impact of climate warming on lake methane emission. Science of The Total Environment 2018, 636, 411-419. https://doi.org/10.1016/j.scitotenv.2018.04.283.
Streese, J.; Stegmann, R. Microbial oxidation of methane from old landfills in biofilters. Waste Management 2003, 23(7), 573-580. https://doi.org/10.1016/S0956-053X(03)00097-7.
Linquist, B.A.; Anders, M.M.,A.; Adviento-Borbe, M.A.; Chaney, R.L.; Nalley, L.L. Reducing greenhouse gas emissions, water use, and grain arsenic levels in rice systems. Global Change Biology 2014, 21(1), 407-417. https://doi.org/10.1111/gcb.12701.
Ribaudo, C.; Bartoli, M.; Longhi, D.; Castaldi, S. CO2 and CH4 fluxes across a Nuphar lutea (L.) Sm. stand. Journal of Limnology 2012, 71(1), e21. https://doi.org/10.4081/mnol.2012.e21.