Biological Oxidation of Dissolved Methane in Palm Oil Mill Biogas Effluents Using an Anoxic Methane-Oxidizing Consortium
Main Article Content
Abstract
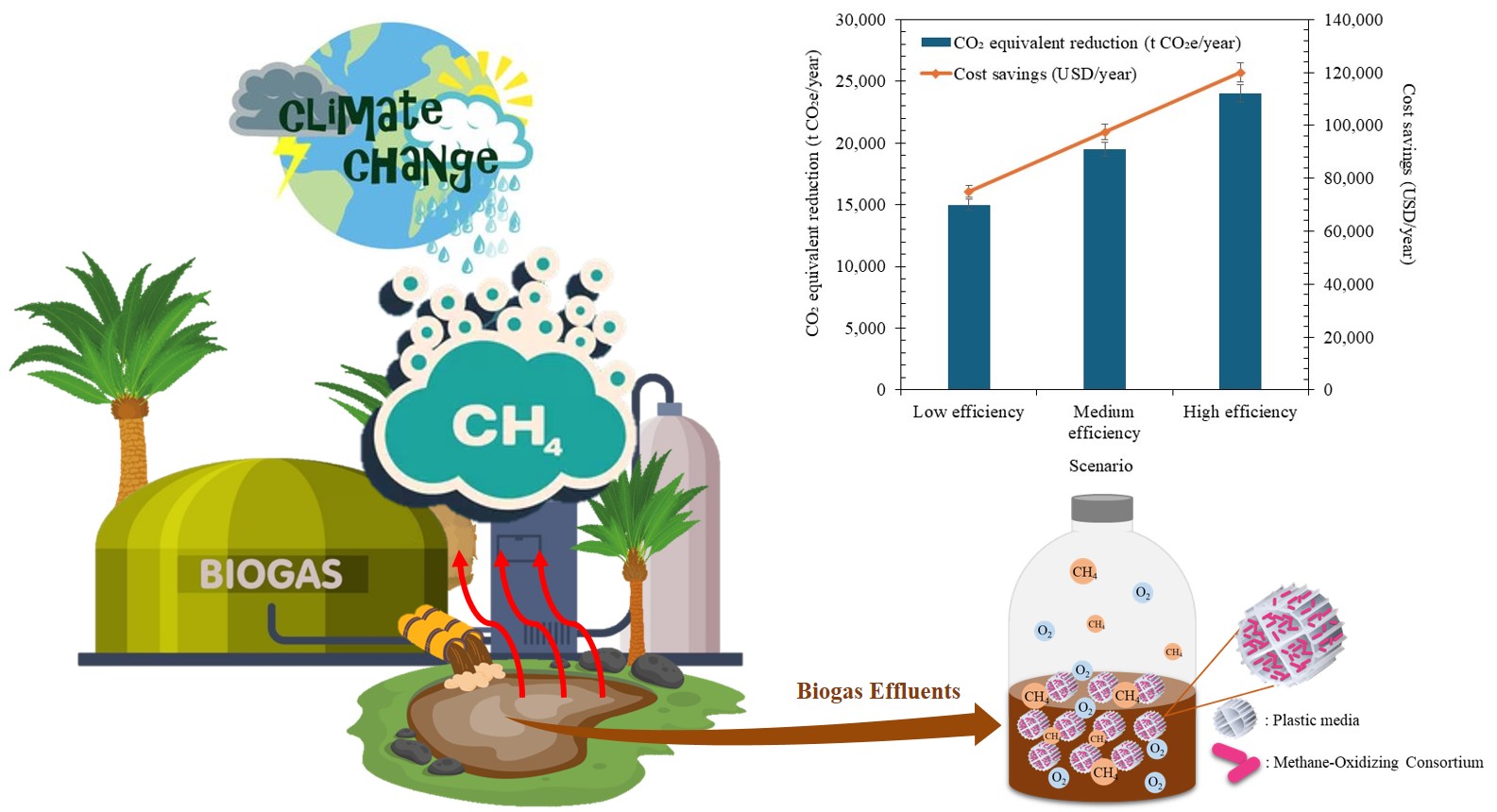
This study investigated the potential of anoxic methane-oxidizing consortia for mitigating dissolved methane in palm oil mill biogas effluents. Microbial consortia from five soil sources were evaluated under various conditions. The cattle farm effluent-derived consortium demonstrated the highest methane reduction efficiency of 76.89% after a 3-week incubation period, with a methane consumption rate of 49.37 mg-CH₄/m²/d. The landfill soil consortium showed the second-highest performance with a 75.48% reduction efficiency under shaking conditions. Environmental factors significantly influenced methane oxidation performance. Optimal conditions were identified as 35°C, pH 7.0, 0.5 mg/L dissolved oxygen, 55 mg/L nitrate concentration, and 5 g/L NaCl. Plastic media enhanced methane reduction efficiency for most microbial sources, particularly for the cattle farm effluent consortium (67.07% efficiency). Characterization of the palm oil mill biogas effluent revealed a COD of 13.15 g/L, BOD of 7.11 g/L, and total Kjeldahl nitrogen of 0.73 g/L. Carbon mass balance analysis confirmed biological methane oxidation, with 45% converted to CO₂, 38% incorporated into biomass, and 12% as dissolved organic carbon. The developed system can potentially mitigate up to 23,067 t CO₂e/year for an average palm oil mill, with associated cost savings of approximately 115,335 USD/year through carbon credits, assuming a credit value of 5 USD/t CO₂e. These findings demonstrate the potential of anoxic methane-oxidizing consortia for greenhouse gas mitigation in the palm oil industry. The study provides insights into optimal conditions and microbial sources for efficient methane oxidation, paving the way for developing effective biological treatment systems for palm oil mill effluents.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
USDA, United States Department of Agriculture. Foreign Agricultural Service: Production – Palm oil. 2024. Available at: https://fas.usda.gov/data/production/commodity/4243000 (accessed on 29 October 2024).
Chaikitkaew, S.; In-Chan, S.; Singkhala, A.; Tukanghan, W.; Mamimin, C.; Reungsang, A.; Birkeland N.K.; O-Thong, S. Clostridium thailandense sp. nov., a novel CO2-reducing acetogenic bacterium isolated from peatland soil. International Journal of Systematic and Evolutionary Microbiology 2022, 72(2). https://doi.org/10.1099/ijsem.0.005254.
O-Thong, S.; Mamimin, C.; Kongjan, P.; Reungsang, A. Two-stage fermentation process for bioenergy and biochemicals production from industrial and agricultural wastewater. Advances in Bioenergy 2020, 5, 249-308. https://doi.org/10.1016/bs.aibe.2020.04.007.
Souza, C.L.; Chernicharo, C.A.L.; Aquino, S.F. Quantification of dissolved methane in UASB reactors treating domestic wastewater under different operating conditions. Water Science & Technology 2011, 64(11), 2259-2264. https://doi.org/10.2166/wst.2011.695.
Cookney, J.; Mcleod, A.; Mathioudakis, V.; Ncube, P.; Soares, A.; Jefferson, B.; McAdam, E. Dissolved methane recovery from anaerobic effluents using hollow fibre membrane contactors. Journal of Membrane Science 2016, 502, 141-150. https://doi.org/10.1016/j.memsci.2015.12.037
Sánchez, A.; Rodríguez-Hernández, L.; Buntner, D.; Esteban-García, A. L.; Tejero, I.; Garrido, J. M. Denitrification coupled with methane oxidation in a membrane bioreactor after methanogenic pre-treatment of wastewater. Journal of Chemical Technology & Biotechnology 2016, 91(12), 2950-2958. https://doi.org/10.1002/jctb.4913
U.S. EPA, United States Environmental Protection Agency, 2020. EPA’s: Air Quality-Cities and Counties. Available online:https://www.epa.gov/air-trends/air-quality-cities-and-counties. (accessed on 27 August 2024).
Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. (Eds.), Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland.
Stazi, V.; Tomei, M.C. Dissolved methane in anaerobic effluents: A review on sustainable strategies for optimization of energy recovery or internal process reuse. Journal of Cleaner Production 2021, 317, 128359. https://doi.org/10.1016/j.jclepro.2021.128359.
Samanta, D.; Sani, R.K. Methane Oxidation via Chemical and Biological Methods: Challenges and Solutions. Methane 2023, 2, 279-303. https://doi.org/10.3390/methane2030019
Guerrero-Cruz, S.; Stultiens, K.; Versantvoort, W. M.; Jetten, M. S.; Kartal, B. Key physiology of a nitrite-dependent methane-oxidizing enrichment culture. Applied and Environmental Microbiology 2019, 85(8). https://doi.org/10.1128/AEM.00124-19.
Costa, R.; Okada, D.; Delforno, T.; Foresti, E. Methane-oxidizing archaea, aerobic methanotrophs and nitrifiers coexist with methane as the sole carbon source. International Biodeterioration & Biodegradation 2019, 138, 57-62. https://doi.org/10.1016/j.ibiod.2019.01.005.
Whittenbury, R.; Phillips, K.C.; Wilkinson, J.F. Enrichment, isolation and some properties of methane-utilizing bacteria. Journal of General Microbiology 1970, 61(2), 205-218. https://doi.org/10.1099/00221287-61-2-205.
Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiological Reviews 1996, 60(2), 439-471. https://doi.org/10.1128/mr.60.2.439-471.1996.
Hernandez, M.E.; Beck, D.A.C.; Lidstrom, M.E.; Chistoserdova, L. Oxygen availability is a major factor in determining the composition of microbial communities involved in methane oxidation. PeerJ 2015, 3, e801. https://doi.org/10.7717/peerj.801.
Zhang, W.; Zhao, Y.; Wang, J.; Gao, Y.; Zhou, L.; Sun, S.; Tang, M.; Peng, Y.; Guo, W.; Wang, H. Effect of nitrate concentration on anaerobic methane oxidation coupled to denitrification in membrane biofilm reactor after prolonged storage. Water, Air, & Soil Pollution 2024, 235, 246. https://doi.org/10.1007/s11270-024-07040-5.
Kalyuzhnaya, M.G.; Gomez, O.A.; Murrell, J.C. The methane-oxidizing bacteria (Methanotrophs). In: McGenity, T. (eds) Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes. Handbook of Hydrocarbon and Lipid Microbiology. 2019. Springer, Cham. https://doi.org/10.1007/978-3-030-14796-9_10.
Ahmadi, F.; Bodraya, T.; Lackner, M. Methane biofiltration processes: A summary of biotic and abiotic factors. Methane 2024, 3(1), 122-148. https://doi.org/10.3390/methane3010008.
Chidambarampadmavathy, K.; Karthikeyan, O.; Huerlimann, R.; Maes, G.; Heimann, K. Response of mixed methanotrophic consortia to different methane to oxygen ratios. Waste Management 2017, 61, 220-228. https://doi.org/10.1016/j.wasman.2016.11.007.
Zheng, X.; Li, H.; Wang, Z.; Sun, Z.; Zhao, L. Intermediates production in methane oxidation coupled with denitrification: Current status, challenges, and future opportunities. Fermentation 2023, 9(7), 645. https://doi.org/10.3390/fermentation9070645.
Reddy, K.R.; Rai, R.; Green, S.; Jyoti, K.C. Effect of pH on methane oxidation and community composition in landfill cover soil. Journal of Environmental Engineering 2020, 146. 04020037. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001712.
Oduor, W.W.; Wandera, S.M.; Murunga, S.I.; Raude, J.M. Enhancement of anaerobic digestion by co-digesting food waste and water hyacinth in improving treatment of organic waste and bio-methane recovery. Heliyon 2022, 8(9), e10580. https://doi.org/10.1016/j.heliyon.2022.e10580.
Bernat, K.; Zaborowska, M.; Zielińska, M.; Wojnowska-Baryła, I.; Ignalewski, W. Biological treatment of leachate from stabilization of biodegradable municipal solid waste in a sequencing batch biofilm reactor. International Journal of Environmental Science and Technology 2021, 18, 1047–1060. https://doi.org/10.1007/s13762-020-02915-6.
Cabrol, L.; Thalasso, F.; Gandois, L.; Sepulveda-Jauregui, A.; Martinez-Cruz, K.; Teisserenc, R.; Tananaev, N.; Tveit, A.; Svenning, M.M.; Barret, M. Anaerobic oxidation of methane and associated microbiome in anoxic water of Northwestern Siberian lakes. Science of The Total Environment 2020, 736, 139588. https://doi.org/10.1016/j.scitotenv.2020.139588.
Kumar, D.J.P.; Mishra, R. K.; Chinnam, S.; Binnal, P.; Dwivedi, N.A comprehensive study on anaerobic digestion of organic solid waste: A review on configurations, operating parameters, techno-economic analysis and current trends. Biotechnology Notes 2024, 5, 33-49. https://doi.org/10.1016/j.biotno.2024.02.001.
Zhao, Y.; Liu, Y.; Cao, S.; Hao, Q.; Liu, C.; Li, Y. Anaerobic oxidation of methane driven by different electron acceptors: A review. Science of The Total Environment 2024, 946, 174287. https://doi.org/10.1016/j.scitotenv.2024.174287.
Meyer-Dombard, D.R.; Bogner, J.E.; Malas, J. A review of landfill microbiology and ecology: a call for modernization with 'next generation' technology. Frontiers in Microbiology 2020, 11, 1127. https://doi.org/10.3389/fmicb.2020.01127.PMID:32582086.
Kim, C.; Walitang, D.I.; Roy Choudhury, A.; Lee, Y.; Lee, S.; Chun, H.; Heo, T.; Park, K.; Sa, T. Changes in soil chemical properties due to long-term compost fertilization regulate methane turnover related gene abundances in rice paddy. Applied Sciences 2022, 12(5), 2652. https://doi.org/10.3390/app12052652.
Govindaraju, A.; Good, N.M.; Zytnick, A.M.; Martinez-Gomez, N.C. Employing methylotrophs for a green economy: One-carbon to fuel them all and through metabolism redesign them. Current Opinion in Microbiology 2022, 67, 102145. https://doi.org/10.1016/j.mib.2022.102145.
Mohammed, S.; Eljack, F.; Al-Sobhi, S.; Kazi, M. A systematic review: The role of emerging carbon capture and conversion technologies for energy transition to clean hydrogen. Journal of Cleaner Production 2024, 447, 141506. https://doi.org/10.1016/j.jclepro.2024.141506.
Sahoo, K.K.; Goswami, G.; Das, D. Biotransformation of methane and carbon dioxide into high-value products by methanotrophs: current state of art and future prospects. Frontiers in Microbiology 2021, 12, 636486. https://doi.org/10.3389/fmicb.2021.636486.
Wilson, R.M.; Tfaily, M.M.; Rich, V.I.; Keller, J.K.; Bridgham, S.D.; Zalman, C.M.; Meredith, L.; Hanson, P.J.; Hines, M.; Pfeifer-Meister, L.; Saleska, S.R.; Crill, P.; Cooper, W.T.; Chanton, J.P.; Kostka, J.E. Hydrogenation of organic matter as a terminal electron sink sustains high CO2:CH4 production ratios during anaerobic decomposition. Organic Geochemistry 2017, 112, 22-32. https://doi.org/10.1016/ j.orggeochem.2017.06.011.
Nguyen, A.D; Chau, T.H.T.; Lee, E.Y. Methanotrophic microbial cell factory platform for simultaneous conversion of methane and xylose to value-added chemicals. Chemical Engineering Journal 2021, 420, 127632. https://doi.org/10.1016/j.cej.2020.127632.
Patel, S.K.; Singh, D.; Pant, D.; Gupta, R.K.; Busi, S.; Singh, R.V.; Lee, J. Polyhydroxyalkanoate production by methanotrophs: recent updates and perspectives. Polymers 2023, 16(18), 2570. https://doi.org/10.3390/polym16182570.
Lu, J.; Yan, W.; Shang, W.; Sun, F.; Li, A.; Sun, J.; Li, X.; Mu, J. Simultaneous enhancement of nitrate removal flux and methane utilization efficiency in MBfR for aerobic methane oxidation coupled to denitrification by using an innovative scalable double-layer membrane. Water Research 2021, 194, 116936. https://doi.org/10.1016/j.watres.2021.116936.
Lu, J.; Shen, Q.; Li, X.; Sun, F.; Yi, J.; Dong, W.; Yan, W.; Du, H.; Mu, J. Surface-manipulated membranes to accelerate biofilm formation and to resist bacterial detachment in MBfR for aerobic methane oxidation coupled to denitrification. Chemical Engineering Journal 2022, 430, 132629. https://doi.org/10.1016/j.cej. 2021.132629.
Pang, S.; Zhang, S.; Lv, X.; Han, B.; Liu, K.; Qiu, C.; Wang, C.; Wang, P.; Toland, H.; He, Z. Characterization of bacterial community in biofilm and sediments of wetlands dominated by aquatic macrophytes. Ecological Engineering 2016, 97, 242-250. https://doi.org/10.1016/j.ecoleng.2016.10.011.
Zheng, Y.; Huang, R.; Wang, B.Z.; Bodelier, P.L.E.; Jia, Z.J. Competitive interactions between methane-and ammonia-oxidizing bacteria modulate carbon and nitrogen cycling in paddy soil. Biogeosciences 2014, 11(12), 3353-3368. https://doi.org/10.5194/bg-11-3353-2014.
Yao, X.; Wang, J.; Hu, B. How methanotrophs respond to pH: A review of ecophysiology. Frontiers in Microbiology 2022, 13. https://doi.org/10.3389/fmicb.2022.1034164.
Gilman, A.; Fu, Y.; Hendershott, M.; Chu, F.; Puri, A. W.; Smith, A. L.; Pesesky, M.; Lieberman, R.; Beck, A. C.; Lidstrom, M. E. Oxygen-limited metabolism in the methanotroph Methylomicrobium buryatense 5GB1C. PeerJ 2017, 5. https://doi.org/10.7717/peerj.3945.
Hu, Z.; Ru, D.; Wang, Y.; Zhang, J.; Jiang, L.; Xu, X.; Nie, L. Optimization of a nitrite-dependent anaerobic methane oxidation (n-damo) process by enhancing methane availability. Bioresource Technology 2019, 275, 101-108. https://doi.org/10.1016/j.biortech.2018.12.035.
Agustiar; Roma; Romanos; Aulia, M.R.; Ramayana. Mass balance of palm waste energy potential in palm oil processing in South West Aceh, Indonesia. IOP Conference Series: Earth and Environmental Science 2024, 129, 012076. https://doi.org/10.1088/1755-1315/1297/1/012076.
Ng, D.K.; Wong, S.L.; Andiappan, V.; Ng, L.Y. Mathematical optimisation for sustainable bio-methane (Bio-CH4) production from palm oil mill effluent (POME). Energy 2023, 265, 126211. https://doi.org/10.1016/j.energy.2022.126211.
IPCC, Climate Change 2023: Summary for Policymakers. In: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, H. Lee and J. Romero (eds.)]. IPCC, Geneva, Switzerland, 2023. pp. 1-34, https://doi.org/10.59327/IPCC/AR6-9789291691647.001.
Jupesta, J.; Akimoto, K.; Boer, R. 2022. Is it possible to achieve carbon neutrality in palm oil production? In: He, BJ., Prasad, D., Pignatta, G., Jupesta, J. (eds) Climate Change and Environmental Sustainability. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-031-12015-2_8.
Mohammad, S.; Baidurah, S.; Kobayashi, T.; Ismail, N.; Leh, C.P. Palm oil mill effluent treatment processes—A review. Processes 2021, 9(5), 739. https://doi.org/10.3390/pr9050739.
Esiri, A.E.; Babayeju, O.A.; Ekemezie, I.O. Implementing sustainable practices in oil and gas operations to minimize environmental footprint. GSC Advanced Research and Reviews 2024, 19(03), 112–121. https://doi.org/10.30574/gscarr.2024.19.3.0207.
Nasution, M.A.; Wulandari, A.; Ahamed, T.; Noguchi, R. Alternative POME treatment technology in the implementation of roundtable on sustainable palm oil, Indonesian Sustainable Palm Oil (ISPO), and Malaysian Sustainable Palm Oil (MSPO) Standards Using LCA and AHP Methods. Sustainability 2019, 12(10), 4101. https://doi.org/10.3390/su12104101.