Optimization of Hydrogen and Methane Co-production from Co-digestion of Canned Seafood Wastewater with Glycerol Waste in a Two-stage Continuous System: Comparing CSTR-PFR and CSTR-CSTR reactors
Main Article Content
Abstract
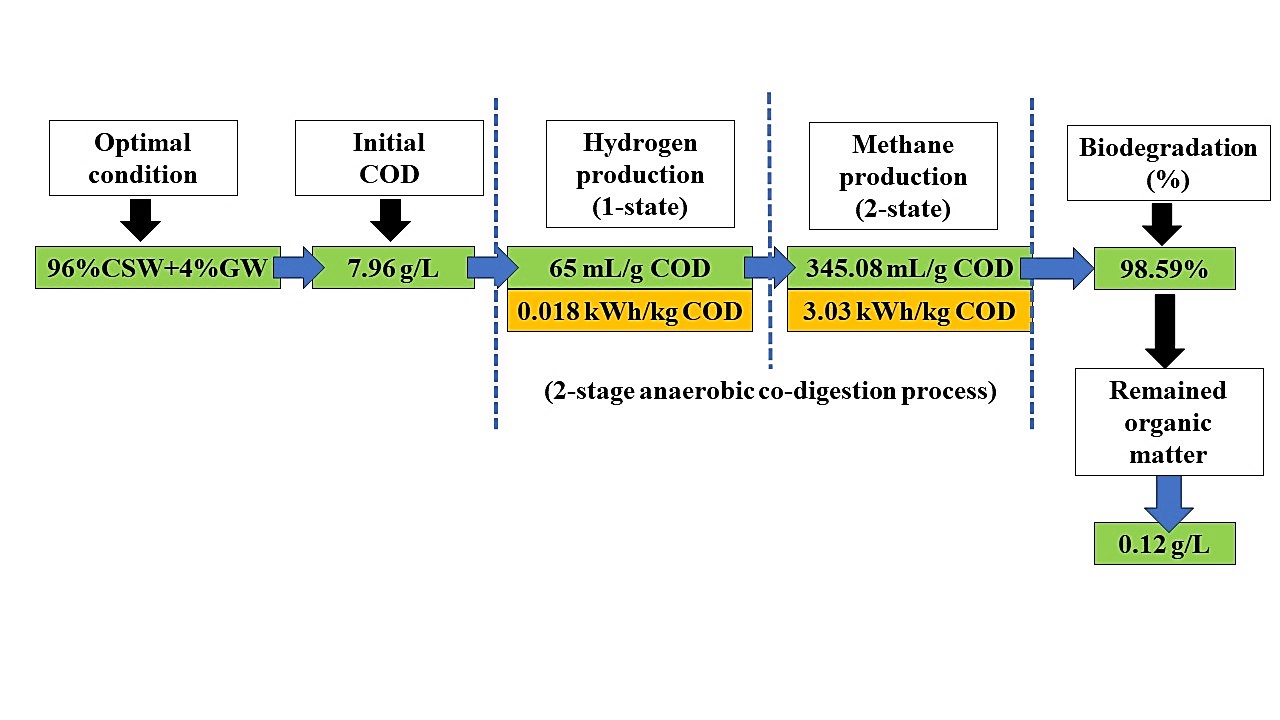
The challenge posed by canned seafood wastewater (CSW) involves a low COD of 6.80 g/L and a high protein concentration of 3.56 g/L, making it unsuitable for hydrogen and methane production. Consequently, the potential return on investment for establishing a commercial system remains inadequate. To address this issue, a two-stage anaerobic digestion system incorporating co-digestion with glycerol waste (GW) was implemented. The two-stage co-digestion of CSW with GW, at various mixing ratios of 99:1, 98:2, 97:3, 96:4, and 95:5% (v/v), resulted in hydrogen yields of 15.6, 33.6, 38.7, 65.0, and 6.3 ml H2/g COD, respectively, while methane yields were measured at 311, 320, 326, 345, and 99 ml CH4/g COD, correspondingly. The ideal conditions for achieving the highest yields of hydrogen and methane from the anaerobic co-digestion of CSW with GW were found to be at a mixing ratio of 96:4% (v/v). The ongoing production of hydrogen and methane in a two-stage process utilizing CSTR-PFR and CSTR-CSTR reactors can yield hydrogen and methane at rates of 27.44 and 163.61 L/L of wastewater, and 20.41 and 145.35 L/L of wastewater, respectively. Anaerobic co-digestion of CSW with GW could enhance the production of hydrogen and methane from a two-stage anaerobic digestion system.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Liu, X.; Li, R.; Ji, M.; Han, L. Hydrogen and methane production by co-digestion of waste activated sludge and food waste in the two-stage fermentation process substrate conversion and energy yield. Bioresource Technol. 2013, 146, 317–323. https://doi.org/10.1016/j.biortech.2013.07.096
Bertasini, D.; Battista, F.; Mancini, R.; Frison, N.; Bolzonella, D. Hydrogen and methane production through two stage anaerobic digestion of straw residues. Environ. Res. 2024, 247, 118101. https://doi.org/10.1016/j.envres.2024.118101
Dong, Z.; Cao, S.; Zhao, B.; Wang, Y.; Wang, L.; Li, N. Optimization of hydrogen-methane co-production from corn stover via enzymatic hydrolysis: Process intensification, microbial community dynamics, and life cycle assessment. Bioresource Technol. 2025, 426, 132367. https://doi.org/10.1016/j.biortech.2025.132367
Sillero, L.; Perez, M.; Solera, R. Optimisation of anaerobic co-digestion in two-stage systems for hydrogen, methane and biofertiliser production. Fuel 2024, 365, 131186. https://doi.org/10.1016/j.fuel.2024.131186
Palenzuela-Rollon, A. Anaerobic Digestion of Fish Processing Wastewater with Special Emphasis on Hydrolysis of Suspended Solids; Taylor and Francis: London, 1999.
Chowdhury, P.; Viraraghavan, T.; Srinivasan, A. Biological treatment processes for fish processing wastewater: A review. Bioresource Technol. 2010, 101(2), 439–449. https://doi.org/10.1016/j.biortech.2009.08.065
Chen, Y.; Cheng, J. J.; Creamer, K. S. Inhibition of anaerobic digestion process: A review. Bioresource Technol. 2008, 99 (10), 4044–4064. https://doi.org/10.1016/j.biortech.2007.01.057
Kangle, K. M.; Kore, S. V.; Kore, V. S.; Kulkarni, G. S. Recent trends in anaerobic co-digestion. Environ. Res. Technol. 2012, 2(4), 210–219.
Yazdani, S. S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18(3), 213–219. https://doi.org/10.1016/j.copbio.2007.05.002
Viana, M. M.; Freitas, A. V.; Leitao, R. C.; Pinto, G. A. S.; Santaella, S. T. Anaerobic digestion of crude glycerol a review. Environ. Technol. Rev. 2012, 1(1), 81–92. https://doi.org/10.1080/09593330.2012.692723
Fountoulakis, M. S.; Manios, T. Enhanced methane and hydrogen production from municipal solid waste and agro-industrial by-products co-digested with crude glycerol. Bioresource Technol. 2009, 100(11), 3043–3047. https://doi.org/10.1016/j.biortech.2009.01.016
Ginkel, S.; Sung, S. Biohydrogen production as a function of pH and substrate concentration. Environ. Sci. Technol. 2001, 35(23), 4726–4730. https://doi.org/10.1021/es001979r
Kongjan, P.; O-Thong, S.; Angelidaki, I. Performance and microbial community analysis of two-stage process with extreame thermophiliclic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Bioresource Technol. 2012, 102(4), 4028–4035. https://doi.org/10.1016/j.biortech.2010.12.009
Altschul, S. F.; Madden, T. L.; Schäffer, A. A.; Zhang, J.; Zhang, Z.; Miller, W.; David, J.; Lipman, D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997, 25(17), 3389–3402. https://doi.org/10.1093/nar/25.17.3389
Yan, Z.; Son, Z.; Li, D.; Yuan, Y.; Liu, X.; Zheng, T. The effects of initial substrate concentration, C/N ratio, and temperature on solid-state anaerobic digestion from composting rice straw. Bioresource Technol. 2015, 177, 266–273. https://doi.org/10.1016/j.biortech.2014.11.089
Mamimin, C.; Thongdumyu, P.; Hniman, A.; Prasertsan, P.; Imai, T.; O-Thong, S. Simultaneous thermophilic hydrogen production and phenol removal from palm oil mill effluent by Thermoanaerobacterium-rich sludge. Int. J. Hydrogen Energy 2012, 37(20), 15598–15606. https://doi.org/10.1016/j.ijhydene.2012.04.062
Symons, G. E.; Buswell, A. M. The methane fermentation of carbohydrates. J. Am. Chem. Soc. 1933, 55 (5), 2028–2036. https://doi.org/10.1021/ja01332a039
Kongjan, K.; O-Thong, S.; Angelidaki, I. Biohydrogen production from desugared molasses (DM) using thermophilic mixed cultures immobilized on heat treated anaerobic sludge granules. Int. J. Hydrogen Energy 2011, 36(22), 14261–14269. https://doi.org/10.1016/j.ijhydene.2011.06.130
Siegert, I.; Banks, C. The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process Biochem. 2005, 40(10), 3412–3418. https://doi.org/10.1016/j.procbio.2005.01.025
Sani, K.; Jariyaboon, R.; O-Thong, S.; Cheirsilp, B.; Kaparaju, P.; Raketh, M.; Kongjan, P. Deploying two-stage anaerobic process to co-digest greasy sludge and waste activated sludge for effective waste treatment and biogas recovery. J. Environ. Manag. 2022, 316, 115307. https://doi.org/10.1016/j.jenvman.2022.115307
Cremonez, P. A.; Teleken, J. G.; Weiser Meier, T. R.; Alves, H. J. Two-stage anaerobic digestion in agroindustrial waste treatment: A review. J. Environ. Manag. 2021, 281, 111854. https://doi.org/10.1016/j.jenvman.2020.111854
Panpong, K.; Srimachai, T.; Nuithitikul, K.; Kongjan, P.; O-Thong, S.; Mai, T.; Kaewthong, N. Anaerobic co-digestion between canned sardine wastewater and glycerol waste for biogas production: Effect of different operating processes. Energy Procedia 2017, 138, 260–266. https://doi.org/10.1016/j.egypro.2017.10.050
Wongarmat, W.; Sittijunda, S.; Imai, T.; Reungsang, A. Co-digestion of filter cake, biogas effluent, and anaerobic sludge for hydrogen and methane production: Optimizing energy recovery through two-stage anaerobic digestion. Carbon Resour. Convers. 2025, 8, 100248. https://doi.org/10.1016/j.crcon.2024.100248
Ting Sun, M.; Lei Fan, X.; Zhao, X.; Fu, S. F.; He, S.; Manasa, M. R. K.; Guo, B. Effects of organic loading rate on biogas production from microalgae: Performance and microbial community structure. Bioresource Technol. 2017, 235, 292–300. https://doi.org/10.1016/j.biortech.2017.03.075
Namsree, P.; Suvajittanont, W.; Puttanlek, C.; Uttapap, D.; Rungsardthong, V. Anaerobic digestion of pineapple pulp and peel in a plug-flow reactor. J. Environ. Manag. 2012, 110, 40–47. https://doi.org/10.1016/j.jenvman.2012.05.017
Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A review on anaerobic co-digestion with a focus on the microbial populations and the effect of multi-stage digester configuration. Energies 2019, 12(6), 1106. https://doi.org/10.3390/en12061106.